New composite may be key to improved hydrogen storage
 (Download Image)
(Download Image)
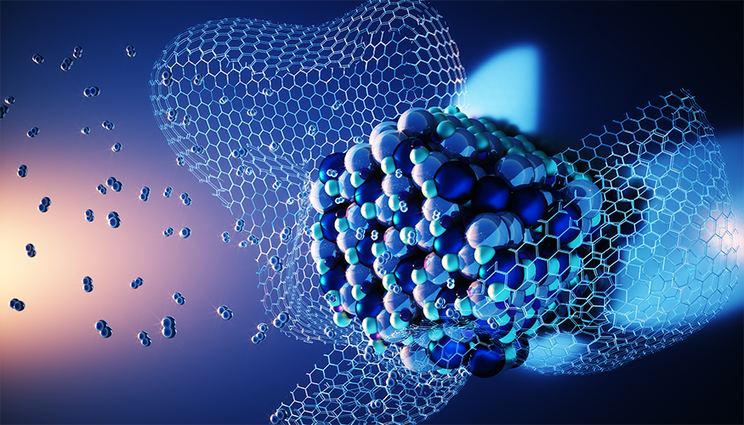
An atomic level view of Li-Mg imide nanoparticle wrapped by a layer of carbon host after hydrogen desorption. Illustration by Ella Maru Studios.
Lawrence Livermore National Laboratory (LLNL) computational scientists worked with experimental collaborators at Lawrence Berkeley and Sandia national laboratories to design metal amide-based composites capable of overcoming key kinetic limitations in their performance as hydrogen storage materials.
Hydrogen possesses the highest energy density of any chemical fuel and can be sustainably produced from a variety of renewable sources. However, storage and transportation of hydrogen is challenging, especially when associated with liquefaction and/or compression.
“Compared to gas or liquid forms, hydrogen storage in solid materials is an attractive alternative, as these materials can offer higher volumetric densities and operate at lower pressures, making them well-suited to meet the evolving needs of renewable energy technologies,” said LLNL scientist Sichi Li, co-first author of a paper to appear on the cover of the journal Advanced Materials Interfaces. “Our goal has been to target metal hydride-based systems, which can store hydrogen reversibly in chemical bonds, then release the H2 gas as needed.”
Magnesium hydride (MgH2) is one of the most desirable hydrogen-storage materials, with high capacity both by weight and by volume. Certain lightweight metal amides, including lithium amide (LiNH2) are likewise promising, and have long been studied by the research community for their potential in hydrogen storage. Unfortunately, when used alone, these materials typically require high temperatures for operation due to slow performance and non-ideal thermodynamic properties. These shortcomings have precluded their use in many of the highest-demand use cases, such as in heavy-duty vehicles.
However, the team discovered that when magnesium hydride is mixed with alkali metal amides and the mixture is incorporated into a porous shell of reduced graphene oxide, a synergistic effect occurs that helps to repair the thermodynamic difficulties and dramatically speed up performance.
Specifically, theoretical calculations by Li and the LLNL team revealed that the graphene oxide facilitates transfer of electrons to the surrounding lithium and hydrogen atoms at the three-way interface of the materials, leading to a weakening of the bonds that hydrogen makes with lithium and nitrogen. As a result, this process enhances the rates of hydrogen regeneration and desorption, as the experimental collaborators were able to successfully demonstrate.
“This is a nice step forward for getting these complex metal hydride materials to work as versatile hydrogen-storage media in high-energy-density applications,” said LLNL scientist Brandon Wood, another author of the study who also leads the hydrogen storage activities at LLNL. “The biggest hurdle has been issues with slow performance, and this work suggests that confined Li-Mg amide composites could hold the key to overcoming this problem. The next step will be to see how we can adjust the compositions through dopants and other additives to possibly further improve performance.”
The research is funded by the Department of Energy, Office of Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cell Technologies Office, through the Hydrogen Materials — Advanced Research Consortium (HyMARC).
Contact
 Anne M. Stark
Anne M. Stark
[email protected]
(925) 422-9799
Related Links
Advanced Material InterfaceHydrogen Material — Advanced Research Consortium
Tags
Advanced Materials and ManufacturingMaterials Science
Physical and Life Sciences
Featured Articles







