Unlocking gas phase uranium oxidation is key to nuclear debris modeling
 (Download Image)
(Download Image)
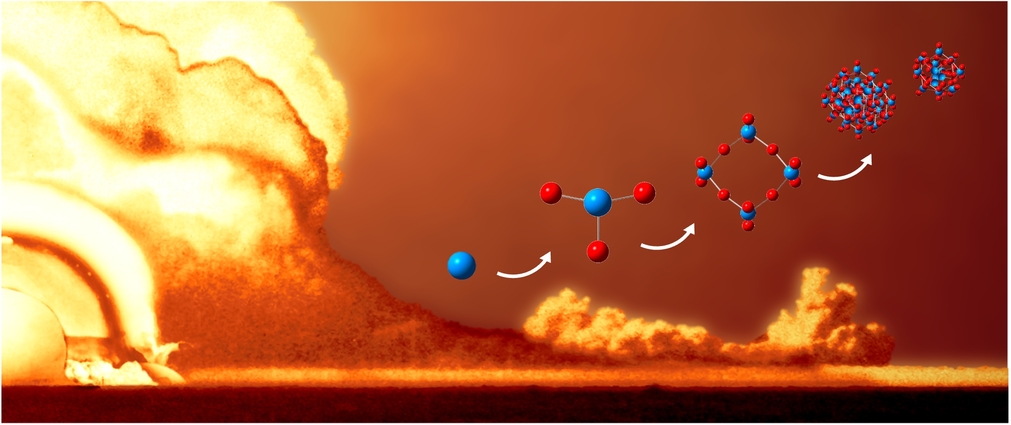
To better understand how nuclear debris forms, Lawrence Livermore National Laboratory scientists have refined the optimization of a uranium oxide reaction mechanism using plasma flow reactor measurements and advanced computational methods. (Image courtesy of Mikhail Finko/LLNL)
In the quest to understand how nuclear debris forms, a team of scientists at Lawrence Livermore National Laboratory (LLNL) has developed an approach to studying the oxidation mechanism of gas phase uranium in extreme environments.
In research recently published in Scientific Reports, the team outlined their work, which combined experimental data from a plasma flow reactor — a unique experimental platform built at LLNL — with advanced computational techniques to shed light on the intricate mechanics of gas phase uranium oxidation at atmospheric pressure.
By employing a Monte Carlo genetic algorithm, a hybrid model that uses random sampling and natural selection, researchers optimized a reaction mechanism based on optical emission spectra measurements from the plasma flow reactor, explained Mikhail Finko, lead researcher on the project. Using this method, reaction rates of gaseous uranium oxidation were then backed out from experimental data instead of being calculated from first principles.
“The methodology we employed not only identifies dominant reaction pathways and rates but also paves the way towards producing a comprehensive, experimentally validated reaction mechanism, a crucial element for modeling nuclear debris formation,” Finko said.
The algorithm LLNL researchers used identified four dominant reaction pathways for forming diatomic uranium oxides and determined the corresponding reaction rates. Furthermore, due to the presence of water in the system, the optimization identified the hydroxyl (OH) radical, a highly reactive chemical species composed of oxygen and hydrogen, as a dominant molecule for producing uranium oxides.
In part due to the development of convenient experimental systems for studying gas phase uranium chemistry, the subject has seen increased interest in recent years. While advances in qualitative understanding of gaseous uranium oxidation mechanics have been made, obtaining validated quantitative reaction rates has remained a challenge, Finko said. The results of this research are directly relevant for the field of material chemistry in extreme environments, as existing reaction mechanisms are not yet well validated.
The work was performed under a Laboratory Directed Research and Development (LDRD) strategic initiative aimed at studying how detonation environments affect the physics and chemistry of nuclear explosions. The LDRD concluded in fiscal year 2022. The research was additionally supported by the Department of Defense’s Defense Threat Reduction Agency.
Other co-authors of the work include Batikan Koroglu, Kate Rodriguez, Timothy Rose, Jonathan Crowhurst, Davide Curreli, Harry Radousky and Kim Knight.
Contact
 Paul Rhien
Paul Rhien
[email protected]
(925) 422-4206
Related Links
Scientific ReportsTags
Advanced Materials and ManufacturingNuclear, Chem, and Isotopic S&T
Defense
Physical and Life Sciences
Strategic Deterrence
Materials Science
Nuclear and Chemical Sciences
Featured Articles







