Pinpointing the effects of nanoconfinement on water
 (Download Image)
(Download Image)
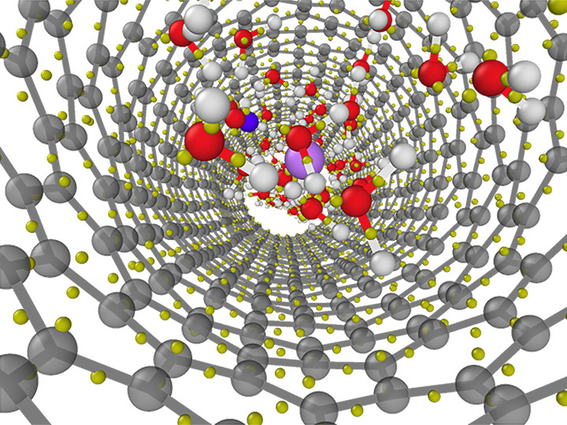
Structure of a lithium chloride solution confined in a 1.1 nanometer diameter carbon nanotube as obtained from first-principles molecular dynamics simulations. Image by Viktor Rozsa/University of Chicago
Researchers have spent decades studying the properties of water and how they change when there are disruptions to their normal behavior. Research on the topic has a wide range of applications, from biochemical systems to water desalination.
A team of scientists from Lawrence Livermore National Laboratory (LLNL), Argonne National Laboratory and the University of Chicago explored how the structure and electronic properties of liquid water can be affected by the presence of ions and nanoconfinement (ions and water confined between material surfaces that are nanometers apart). They employed first-principle simulations to search for signs of these perturbations. The research appears in the Journal of Chemical Physics.
For comparison, the team, led by lead author Viktor Rozsa, a graduate student at the University of Chicago and a DOE NNSA Stewardship Science Graduate fellow, performed simulations for water inside semiconducting nanotubes with diameters of 1.1 and 1.5 nanometers, respectively (one nanometer is about 17,000 times smaller than a human hair). They discovered that due to the nanoconfinement, there are competing effects of broken hydrogen bonds and water–carbon interactions on the molecular polarizability. They identified water molecular polarizability as the “fingerprint” of ion and nanoconfinement perturbation.
“The molecular polarizabilities displayed a competing balance between reductions from structure breaking and enhancements at the interface from the nanotube,” said LLNL’s Anh Pham, a materials scientist in the Quantum Simulations Group.
This work can be extended to understand the effect of anions or divalent ions on confined water. In the future, the researchers aim to study how the competing effects on molecular polarizability are influenced by other solvated ions and in different degrees of confinement.
“Our results highlight the importance of the inclusion of polarizability for realistic simulations of water in complex environments and may assist with parameterization of future interatomic potentials,” said Giulia Galli, the Liew Family Professor in the Pritzker School of Molecular Engineering at the University of Chicago, senior scientist at Argonne and a co-author of the study.
The paper describing this research was chosen as a featured paper by the Journal of Chemical Physics and also was highlighted by the American Institute of Physics.
The research was conducted at two Energy Frontier Research Centers, Advanced Materials for Energy-Water Systems and the Center for Enhanced Nanofluidic Transport. The project was funded by the DOE Office of Science and the LLNL Grand Challenge Program.
Contact
 Anne M. Stark
Anne M. Stark
[email protected]
(925) 422-9799
Related Links
Journal of Chemical PhysicsAmerican Institute of Physics
Advanced Materials for Energy-Water Systems (AMEWS)
Center for Enhanced Nanofluidic Transport (CENT)
Tags
Advanced Materials and ManufacturingMaterials Science
Physical and Life Sciences
Featured Articles