New chemistry leads to more robust carbon capture materials
 (Download Image)
(Download Image)
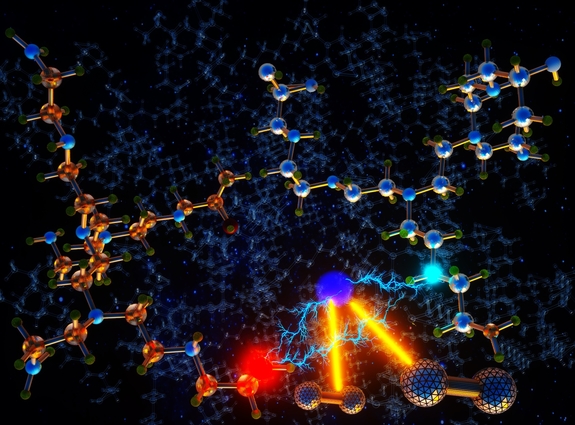
Conceptual illustration demonstrating the antioxidative impact of epoxide-amine hydrogen bonding on aminopolymer-based direct air capture adsorbents. Image courtesy of Ella Maru Studio.
In a significant stride toward implementing scalable climate solutions, Lawrence Livermore National Laboratory (LLNL) scientists have uncovered how some carbon capture materials have improved lifetime compared to others. These materials are key in addressing greenhouse gas emissions and global warming concerns.
Researchers have shed light on the mechanism that empowers these materials, extending their operational lifespan and effectiveness in capturing harmful carbon dioxide (CO2) emissions. This research is featured on the cover of Chemical Communications.
Carbon-capture technologies are carbon-absorbing agents known as poly(ethylenimine) (PEI) sorbents. However, a persistent challenge has been the waning CO2-capturing capability of these sorbents over time that is caused by degradation.
A collaborative team from LLNL and the National Renewable Energy Laboratory (NREL) delved into the concept of epoxide-functionalization, a method designed to counteract the diminishing effectiveness of these materials.
“While the term may sound intricate, it involves a simple one-pot reaction to introduce new functional groups into the sorbents,” said LLNL scientist Sichi Li, lead and co-corresponding author of the paper. “Through meticulous computer simulations, we found that these functional groups foster robust hydrogen bonds within the material's structure and slow down undesired oxidation reactions, enhancing its lifetime.”
The outcomes of real-world CO2 capture experiments aligned with those from virtual simulations. The enhanced sorbents demonstrated exceptional durability, retaining their CO2-capturing capacity even after multiple cycles of use.
“This finding marks a notable step toward enhancing our ability to understand and design novel long-lived sorbents for direct air carbon capture, allowing these materials to meaningfully contribute to combating climate change,” said Simon Pang, co-corresponding author and project principal investigator.
But this is just the beginning. Researchers are already exploring new chemical modifications to create sorbents with even stronger bonds, promising to amplify the impact of carbon capture technologies and move us closer to a greener world.
LLNL co-authors also include Marcos Calegari Andrade, Anthony Varni, Elwin Hunter Sellars and Maxwell Marple. The research is funded by the Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division. Computational resources are provided by the LLNL institutional grand challenge program.
Contact
 Anne M. Stark
Anne M. Stark
[email protected]
(925) 422-9799
Related Links
Chemical CommunicationsDepartment of Energy, Office of Science, Basic Energy Sciences
Tags
Advanced Materials and ManufacturingPhysical and Life Sciences
Materials Science
Office of Science
Science
Featured Articles







