Matter at extremes: a question of scale
Scaling laws are like a secret code used across science to break down complex phenomena into simple mathematical expressions. These equations help us to understand how one factor in a system relates to other factors that determine the system’s behavior. For example, Kleiber’s Law, one of the best-known scaling laws, observes that metabolic rates of many organisms — from mice to elephants, and humans even — scale with body mass to the ¾-th power. This simplicity hints at an elegant underlying law in the phenomena being studied.
In a paper published last week by Physical Review Letters, a team of researchers from Lawrence Livermore National Laboratory (LLNL), Sandia National Laboratories and UC Davis, have developed a scaling law to analyze the kinetics of high-pressure, rapid solidification of metastable liquids observed in national laboratory and academic experiments over the past few decades.
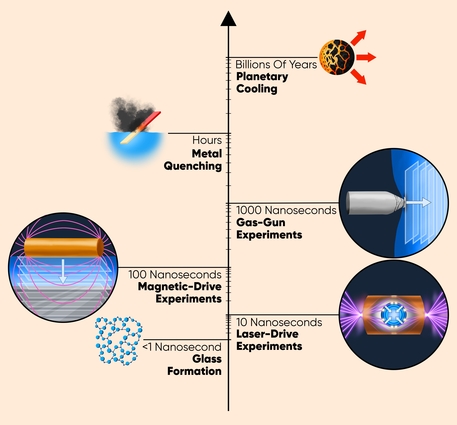
The temperature and pressure conditions under which phase transitions such as solidification occur are given by “maps” called phase diagrams. These maps apply only under equilibrium conditions, meaning that the transition is given enough time to fully occur. The experiments analyzed by the team, however, use a dynamic-compression platform to rapidly compress liquids in just tens to thousands of nanoseconds. This high-speed compression allowed them to explore exotic liquid states that solidify at pressures that lie far beyond what phase diagrams predict for equilibrium conditions.
A key observation from these experiments that has puzzled researchers is the seemingly automatic ability of this nonequilibrium solidification process to “renormalize” itself to match the timescale of the experiments. That is, it has been observed that even if the compression rate of the experiment is varied by a factor of 100 over the same compression path, the solidification kinetics correspondingly get scaled by roughly the same factor of 100.
“This renormalization is why the same phase transition that is observable on the longer timescale of gas-gun experiments [~1000 nanoseconds] also is observable on the much shorter timescale of laser-drive experiments [~10 nanoseconds], such as those conducted at the National Ignition Facility, making them both valuable platforms for studying solidification kinetics,” said Philip Myint, a staff scientist in LLNL’s Physics Division and an author of the paper.
Through a combination of experiments, theory and computational simulations, the team has derived a scaling law that explains and quantifies this compression-rate dependence across different experimental platforms. The scaling law is a culmination of several years of both theoretical and experimental study across a variety of systems; the published modern experimental data on solidification under dynamic compression, most of which pertain to water; and the team’s own experiments conducted on gallium at the Thor pulsed-power machine at Sandia.
The new gallium experiments play a crucial role in the research because gallium and water are very different materials. For example, gallium is a monatomic metal, while water is a molecular insulator. This scaling law, developed using data from both gallium and water, is expected — perhaps after some fine-tuning as more data becomes available — to be adaptable to a wide range of materials, Myint said. To help generalize their scaling law to cover a broad range of materials, the team applied dimensional analysis, a time-tested technique that has been used to develop many of the scaling laws throughout history.
With further testing and refinement, the new scaling law may allow researchers to predict rapid solidification in various materials without needing experiments or simulations. It also invites important questions, like whether similar scaling patterns apply to other high-pressure phenomena, such as solid-to-solid changes or melting under extreme compression. In their paper, the team also highlighted an interesting application — using the scaling law to determine the melt temperature of materials through dynamic-compression experiments.
“Obtaining melt temperatures under dynamic compression is a long-standing area of challenge in high-pressure science that is fundamental to understanding and modeling the evolution of planetary interiors,” said Dane Sterbentz, a staff scientist in the Materials Science Division (MSD) who works on analyzing such experiments through computer models.
“Being able to understand and control such properties is a necessary step toward enabling the long-term goal of engineering and synthesizing novel materials via high-pressure techniques,” said Jon Belof, a group leader in MSD who leads LLNL’s Phase-Transition Kinetics Program.
Other authors on the paper include Justin Brown and Brian Stoltzfus from Sandia and Jean-Pierre Delpanque, professor of mechanical & aerospace engineering and dean of graduate studies at UC Davis.
The work was conducted with funding from the Physics and Engineering Models (PEM) element of the Advanced Simulation and Computing (ASC) Program, as well as the Laboratory Residency Graduate Fellowship (for Sterbentz) from the DOE/NNSA.
Contact
 Paul Rhien
Paul Rhien
[email protected]
(925) 422-4206
Tags
Advanced Materials and ManufacturingLasers and Optical S&T
National Ignition Facility and Photon Science
Physical and Life Sciences
Lasers
Materials Science
Physics
Science
Featured Articles