Platform tests colorectal cancer therapies in vitro
 (Download Image)
(Download Image)
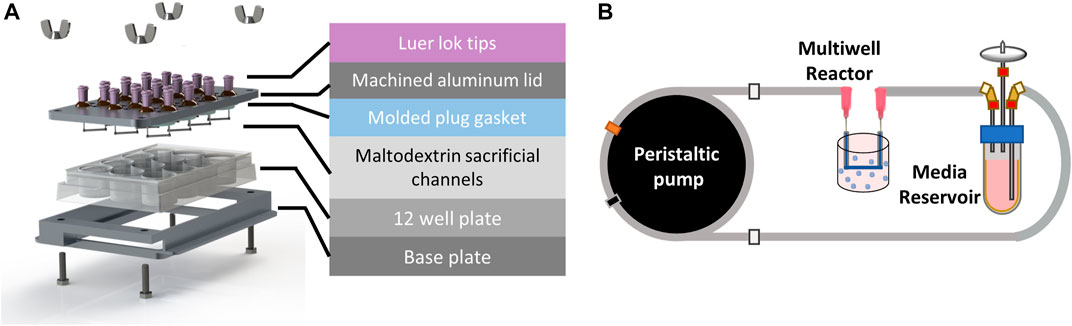
An exploded view of the perfused multi-well bioreactor platform, designed to utilize a 12-well tissue culture plate for ease of handling and fabrication. Patient-derived organoids (sampled patient tissue) were prepared and placed into each well.
Colorectal cancer (CRC) — cancer of the colon or rectum — is the third-most common cancer in both men and women in the United States and the second-most common cause of cancer-related death in developed countries.
Although surgery is highly successful for patients with a localized disease or disease confined to a narrow region (stages I–III), a total of 60% of CRC patients are diagnosed with Stage IV liver metastasis (CRCLM), for which surgery is insufficient. While combination regimes of chemotherapeutic agents and targeted therapies benefit some patients, the five-year relative survival rate for metastatic CRC remains just 14%. Thus, there is an urgent need to develop additional therapies for CRCLM.
In fact, patient-derived organoids (PDOs) — sampled patient tissue — are emerging as reliable preclinical tools to develop and test therapies for metastatic CRC. PDOs have been shown to mimic drug response behavior of the tumor from which they are derived and retain genomic and some phenotypic characteristics of the original tumor. Therefore, PDOs can be used as models to understand patient-specific drug responses and investigate cancer cell growth.
However, because studying drug transport in tumors in vivo is complex, in vitro platforms are also needed to better understand tumor progression and treatment resistance. In support of this need, Lawrence Livermore National Laboratory scientists and collaborators have developed a platform capable of studying CRCLM PDO response to various chemotherapeutic gradients. Their research is detailed in a recent Frontiers in Bioengineering and Biotechnology paper.
The team’s perfused multi-well bioreactor platform was designed around a standard 12-well cell culture plate to minimize the number of custom parts and enable easy assembly. This layout provides 12 independently fed wells that can be used to study multiple drug concentrations or combinations in one experiment.
When testing this platform, the team investigated how tumor organoids respond to a common cancer drug treatment, 5-fluorouracil (5-FU), in varying environments. Patient-derived colorectal liver metastasis organoids were cultured in the multi-well bioreactor platform and organoid response was compared to organoids cultured in media and static hydrogel, which are two common PDO culture models routinely used for drug testing. Researchers ran computer simulations prior to testing to determine how the drug would be distributed in each platform.
The simulation verified that after seven days of exposure to 5-FU in the bioreactor, a concentration gradient formed, reaching a higher average concentration close to the channel (region 1) and a lower average concentration far from the channel (region 2). This gradient correlates to a lower viability in region 1 than region 2. The simulation results helped ensure the cumulative dose the organoids received for the duration of the experiment were comparable across platforms.
Despite the organoids in each condition receiving comparable cumulative doses of drugs, organoids cultured in media had a significantly lower viability, suggesting that cumulative dose alone does not explain differences in cell viability and that culturing PDOs in a 3D hydrogel influences drug response. Additionally, organoids in media were immediately exposed to 5-FU while the static gel and bioreactor were exposed slowly as the drug diffused through the hydrogel.
“Our results demonstrate the platform’s ability to generate gradient conditions and test multiple drug concentrations on PDOs at once while also highlighting the importance of using transport simulations to properly assess culture environment and obtain accurate interpretations of drug response,” said LLNL scientist Elisa Wasson. “Using our platform and transport modeling, flow parameters may be tuned to control these gradients and enable testing of a range of therapeutic agents and different dosing strategies.”
Future studies could utilize the platform to investigate how other microenvironmental gradients such as oxygen or tumor promoting soluble factors influence PDO drug response. Additionally, tumor-immune interactions may be investigated by circulating immune cells through the channel and observing invasion into the surrounding PDOs loaded hydrogel.
This research was supported by LLNL’s Laboratory Directed Research Development program.
—Shelby Conn
Contact
 Anne M. Stark
Anne M. Stark
[email protected]
(925) 422-9799
Tags
Bioscience and BioengineeringBiosciences and Biotechnology
Advanced Materials and Manufacturing
Materials Science
Physical and Life Sciences
Featured Articles







