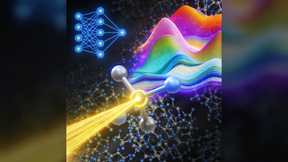
Ion–water clusters in carbon nanotubes
 (Download Image)
(Download Image)
The cover image depicts an isolated ion–water cluster inside a carbon nanotube. Such clusters are responsible for the unusually fast electrophoretic transport of potassium ions and lead to a strong breakdown of the Nernst–Einstein relation.
In 1888, Walther Nernst proposed a universal relation between a charged particle’s electrophoretic mobility (a solute’s velocity in response to an applied electric field) and its diffusion coefficient (the rate at which a particle free-diffuses through medium). The microscopic origins of this relation were revealed in 1905 by Albert Einstein. These works cemented the recognition that the microscopic motion of a particle is determined by random collisions with molecules of the surrounding medium, regardless of the macroscopic (visible) forces that drive that motion. Known as the Nernst–Einstein (NE) relation, this phenomenon is an essential building block of several important theories of ion transport that describe ion movement in a solution or across a membrane.
The NE relation holds extremely well for a variety of dilute electrolyte solutions (water and salt ions). Yet, most of the existing work on ion transport takes the NE relation for granted. In fact, there are very few measurements of ion mobility and ion diffusion in the same nanochannel that would serve as a rigorous test for the applicability of the NE relation, especially under the strong confinement of nanoscale channels.
In a recent study, featured on the February 2023 cover of the journal Nature Nanotechnology, LLNL scientists measured the diffusion and electromigration of potassium ions (K+) in narrow 0.8-nanometer-diameter single-walled carbon nanotube porins (CNTPs)—short segments of single-wall carbon nanotubes that mimic the basic functionality of biological membranes and have applications in areas such as direct drug delivery and water desalination. For the experiments, the CNTP nanochannel was inserted in a lipid bilayer, with a potassium chloride (KCl) electrolyte solution on both sides. To better understand the experimental setup, imagine that the CNTP is a bridge, the lipid bilayer is a body of water, the K+ ions are the cars traveling from one side to another, and the KCl represents the two pieces of land that the bridge connects.
Their measurements demonstrated that the extreme spatial confinement in these pores greatly hinders the diffusion of K+, reducing the diffusion coefficient by three orders of magnitude (103). Surprisingly, the electrophoretic mobility was largely unaffected, leading researchers to conclude that the NE relation was completely broken down.
To determine the origin of the breakdown, LLNL collaborators at the Massachusetts Institute of Technology conducted molecular dynamics simulations, finding that the overall relation breaks down because of the two distinct mechanisms for ion diffusion and electromigration (concentration-gradient-driven versus electric-field-driven) that exist inside these nanotubes. That is, in concentration-gradient-driven ion transport, ions were found to diffuse slowly in the presence of a single-file water chain; but under external electric fields, the water breaks up, and ions transport in the form of ion-water clusters. These findings demonstrate the unique nature of transport in this nanofluidic regime, and highlight the importance of separately measuring the ion diffusion coefficient and electrophoretic mobility in biological and artificial channels, especially under extreme nanoscale confinement.
[Z. Li, R.P. Misra, Y. Li, Y-C. Yao, S. Zhao, Y. Zhang, Y. Chen, D. Blankschtein, and A. Noy, Breakdown of the Nernst–Einstein relation in carbon nanotube porins, Nature Nanotechnology (2022), doi: 10.6084/m9.figshare.20728327.]
Tags
Advanced Materials and ManufacturingMaterials Science
Physical and Life Sciences
Featured Articles