Metal ion solvation in ionic liquids
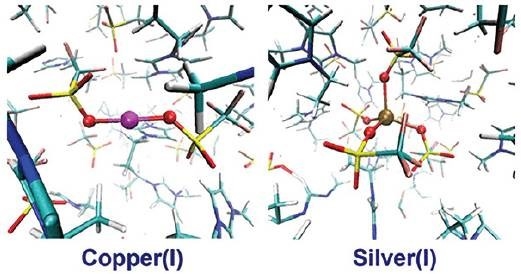
Understanding the behavior of metal ions in room temperature ionic liquids is essential for predicting and optimizing performance for technologies like metal electrodeposition. A recent paper by Livermore researchers describes a first-principles molecular dynamics simulations approach to understanding and comparing the key structural properties metal ions (Cu+ and Ag+) in a common room-temperature ionic liquid. The scientists show that, when compared to Cu+, the larger Ag+ shows a more disordered and flexible solvation structure with more frequent exchange of the ionic liquid species between its solvation shells. They are using this new information to help understand experimental electrodeposition results.
This research was funded by the Laboratory Directed Research and Development Program (17-ERD-047).
[T.A. Pham, C. Horwood, A. Maiti, V. Peters, T. Bunn, and M. Stadermann, Solvation Properties of Silver and Copper Ions in a Room Temperature Ionic Liquid: A First-Principles Study, J. Phys. Chem. B 122 (50), 12139–12146 (2018), doi: 10.1021/acs.jpcb.8b10559.]
Tags
Bioscience and BioengineeringBiosecurity & Bioscience
Advanced Materials and Manufacturing
Materials Science
Physical and Life Sciences
Featured Articles