Reinventing the soft X-ray spectroscopy of actinides
 (Download Image)
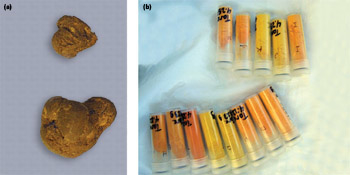
(a) A sample of uranium oxide; (b) Suite of known uranium oxide
(Download Image)
(a) A sample of uranium oxide; (b) Suite of known uranium oxide
Using LLNL's new Fano/Resonant Inverse Photoelectron Spectroscopy (RIPES) spectrometer, Lab researchers Jim Tobin and Sung Woo Yu conducted a series of experiments on cerium oxide and uranium dioxide. The duo also looked at the X-ray absorption spectroscopy of uranium dioxide and the X-ray photoelectron spectroscopy of uranium dioxide.
"We demonstrated that we can directly determine and separate the electronic structure in the unoccupied states of an actinide without the need to go to a synchrotron radiation source," Tobin said.
The actinides are composed of the 15 metallic chemical elements on the periodic table with atomic numbers from 89 to 103, actinium through lawrencium.
The next step for the LLNL team is to solve a mystery that has plagued scientists for more than 65 years: What is the electronic structure of plutonium (Pu), and how does electron correlation in Pu work?
Over the last 60 years, the mystery of Pu's electronic structure has been unraveled slowly. In a series of experiments and linked theoretical modeling, the range of possible solutions for plutonium's electronic structure has been reduced dramatically.
"Our goal is to resolve the Pu electronic structure enigma," Tobin said. "If we are able to put a Pu sample in the Fano/RIPES spectrometer, we should be able to do just that."
The research appears in the Oct. 14 issue of the journal, Physical Review Letters.
Contact
Anne M Stark[email protected]
925-422-9799
Tags
Physical and Life SciencesFeatured Articles







