Microbial research unravels a global nitrogen mystery
 (Download Image)
(Download Image)
LLNL scientist Xavier Mayali used the LLNL nanoSIMS analysis instrument to quantify the flux of ammonia and urea into the cells. Photo by Blaise Douros/LLNL.
Ammonia-oxidizing microorganisms (AOM) use ammonia for energy and account for the annual oxidation of approximately 2.3 trillion kilograms of nitrogen in soil, freshwater, the subsurface and man-made ecosystems.
But one major question that has remained unanswered for decades is how different AOM species coexist in the same environment: do they compete for ammonia or instead use other alternative compounds for their energy needs?
New research by Lawrence Livermore National Laboratory (LLNL), the University of Oklahoma and other collaborators found an answer that significantly changes the understanding of ammonia oxidation, a critical component of the global nitrogen cycle. The research appears in Nature Microbiology.
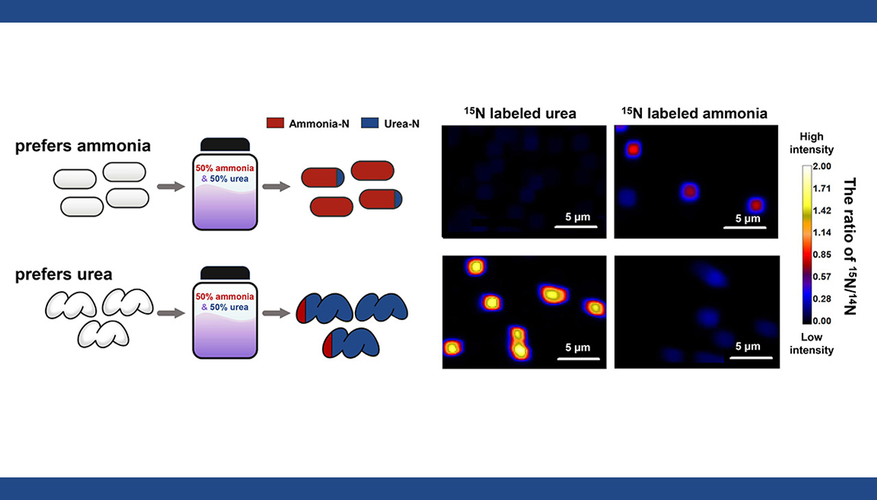
“The different lineages of AOM are simultaneously growing in the same environment and were thought to simply compete for ammonia,” said Wei Qin, a University of Oklahoma assistant professor and lead author of the paper. “Our collaborative research focused on determining why and how these metabolically conserved lineages are able to coexist without direct competition, and we examined their abilities to use another compound when ammonia was present.”
More than half of the AOM species also are adapted to utilize urea, a widely available organic nitrogen compound that accounts for ~40% of all nitrogen in fertilizers, as an alternative energy source. Urea, however, requires AOM to use an additional amount of energy because it is a more complex molecular structure and needs to first be broken down into ammonia inside the AOM cells before further utilization. Knowing this, Qin’s team and their collaborators sought to understand how AOM acquire and metabolize ammonia and urea when both are available simultaneously.
“We always called urea an alternative substrate to ammonia,” Qin said. “Now, we realize that a major lineage of AOM actually prefer urea and repress the use of ammonia when urea is present. This discovery challenges dominant assumptions that had persisted for more than 100 years since the cultivation of the first AOM species.”
LLNL scientists helped design the experiments to measure how much nitrogen the different AOM were getting from ammonia and from urea, and then used the LLNL nanoSIMS analysis instrument to quantify that flux into the cells. The key was to provide the two substrates simultaneously but label one substrate isotope with Nitrogen-15, but not the other (and then vice-versa).
LLNL scientist and co-author Xavier Mayali explained the research this way: “Most people eat their main dish and dessert separately, and it is not always obvious if they prefer one over the other. What if we gave the main dish and the dessert to them at the same time and tested which one they eat more? Here, we used isotopes to test if the AOM cells preferred urea or ammonia when both were equally available, similar to this concept of providing two dishes to someone eating dinner. We found that some AOM preferred ammonia and others preferred urea.”
The research findings show that different AOM lineages employ different regulatory strategies for ammonia or urea use, thereby minimizing direct competition with one another and allowing for coexistence. These differential preferences reveal a hidden physiological biodiversity of this globally important functional group and have real-world consequences that will need to be explored further, researchers said.
Other LLNL scientists include Peter Weber, who heads the nanoSIMS laboratory, and former postdoc Julie Johnston. Other collaborators include researchers from the University of Washington, the University of Florida, Princeton University, Xiamen University and Nanjing Agricultural University.
This work was funded by the U.S. Department of Energy’s Office of Science, Division of Biological and Environmental Research, the Defense Advanced Research Projects Agency, the Department of Agriculture National Institute of Food and Agriculture, Simons Foundation, the National Natural Science Foundation of China and others.
Contact
 Anne M. Stark
Anne M. Stark
[email protected]
(925) 422-9799
Related Links
Nature MicrobiologyUniversity of Oklahoma
University of Washington
University of Florida
Princeton University
Xiamen University
Nanjing Agricultural University
Division of Biological and Environmental Research
Department of Agriculture National Institute of Food and Agriculture
National Natural Science Foundation of China
Tags
Bioscience and BioengineeringPhysical and Life Sciences
Nuclear and Chemical Sciences
Office of Science
Soil microbiome sfa
Featured Articles