Microbes at work cleaning up the environment
LIVERMORE, Calif. – It may sound counterintuitive to use a microbial protein to improve water quality.
Butsome bacteria are doing just that to protectthemselves from potentially toxic nanoparticlesin their own environments, and clean up crewsof the future could potentially do the samething on a larger scale.
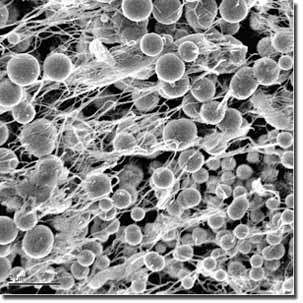
A team from Lawrence Livermore National Laboratory,UC Berkeley and Lawrence Berkeley NationalLaboratory found that bacteria from an abandonedmine excrete proteins that cause metal nanoparticlesto aggregate. The bacteria are binding andimmobilizing the metals in the nanoparticlesand the nanoparticles themselves, which arepotentially toxic to the bacteria.
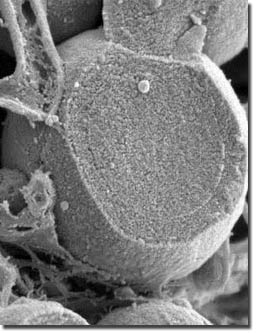
Sulfate-reducingbacteria can cause heavy metals such as zinc(Zn) to precipitate and form nanoparticles.However, these particles are able to movefreely because they are so small (typically2-6 nanometers in diameter) and can redissolveif conditions change.
Inthe case of the mine bacteria, the researchersshowed that the bacteria are causing thenanoparticle aggregation, thereby protectingthemselves. When the metal nanoparticlesaggregate, they don’t move as easilyand are less soluble.
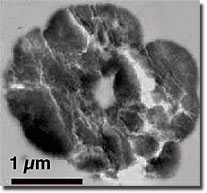
Usingsecondary ion mass spectrometry, transmissionelectron microscopy and infra-red spectroscopy,the scientists were able to study whetherprotein contributes to the formation of denselyaggregated nanoparticulate zinc sulfide spheroids.
They also studied whether various amino acidsinduce rapid aggregation in metal-sulfidenanoparticles.
The answer was yes in both cases.
"Thisdemonstrates an extracellular biomineralizationmechanism that is unexpected because it involvesthe bacteria excreting proteins for nanoparticleaggregation away from the cells," saidPeter Weber, one of the LLNL authors of thepaper appearing in the June 15 edition ofthe journal Science .
Weberand LLNL colleague Ian Hutcheon used LLNL’sNanoSIMS (high- resolution secondary ionmass spectrometer) to study the metal-sulfidenanoparticle aggregation in sulfate-reducingbacteria dominated biofilms collected fromthe Piquette Mine, a flooded system in southwesternWisconsin.
The team found that organic nitrogen was highly concentrated in all of the zinc-sulfide aggregates, indicating a high protein or polypeptide content relative to inorganic zinc-sulfide minerals. In combination with the other techniques and experiments, the team concluded that the protein caused the zinc-sulfide nanoparticle aggregation.
Theresearchers conducted experiments guidedby known bacterial metal-binding proteinsthat bind zinc and other potentially toxicmetals at cysteine locales. Cysteine is asulfur-containing amino acid. Amino acidsare the building blocks of proteins.
Theresearchers found that inorganic aggregationof zinc-sulfide initially occurred rapidlyto 100-nanometer diameter aggregates butthen slowed or ceased after one week. However,zinc-sulfide nanoparticles in the presenceof cysteine displayed more extensive andprolonged aggregation, accumulating up to1-10 micron (1/1000th of a millimeter)-sizedstructures.
"Potentiallywe can use cysteine or cysteine-rich polypeptidesor proteins for nanoparticle clean up," Webersaid. "With the boom in nanoscience,people are naturally asking questions aboutthe potential environmental impacts. Here,we see that naturally produced nanoparticlescan be naturally controlled."
Foundedin 1952, Lawrence Livermore National Laboratoryis a national security laboratory, with amission to ensure national security and applyscience and technology to the important issuesof our time. Lawrence Livermore NationalLaboratory is managed by the University ofCalifornia for the U.S. Department of Energy’sNational Nuclear Security Administration.
Contact
Anne M. Stark[email protected]
925-422-9799
Related Links
Decoding the Origin of a BioagentSolving a Nitrogen Fixation Conundrum with NanoSIMS
Research advances understanding of how cell membranes function
Lawrence Berkeley National Lab news release
Tags
NanoSIMSFeatured Articles