Modifying the molecular structure of carbon nanotubes
Carbon nanotubes (CNTs) are known for their high tensile strength and electrical and thermal conductivities, making them ideal for a wide range of consumer applications—energy storage, electronics, etc. However, current approaches to CNT synthesis are limited in their ability to control the placement of atoms on the surface of nanotubes. Some of these limitations stem from a lack of understanding the chemical bond-building mechanisms at play in CNT growth.
An improved understanding of the mechanisms used for CNT synthesis could simultaneously lower cost, enhance control, and reduce environmental impacts of production. Especially since CNT manufacturing remains an energy intensive and inefficient process, requiring 2–100 times more energy than aluminum production and consuming resources at waste ratios equivalent to the pharmaceutical industry.
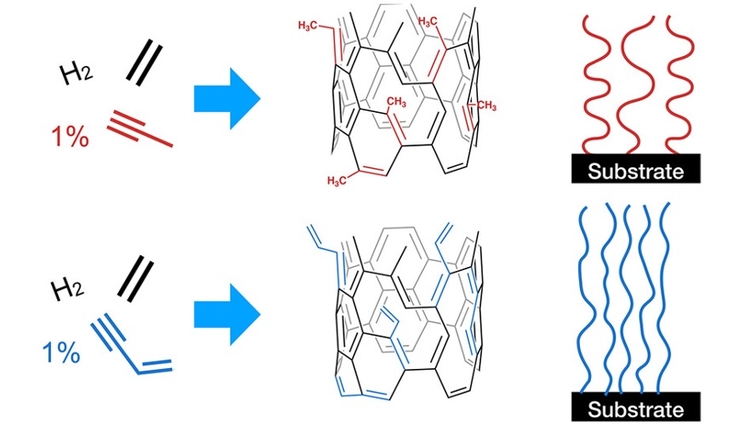
As a proof-of-concept demonstration, BBTD scientists and collaborators have illustrated how varying the main alkyne precursors can be leveraged to alter CNT structure and bonding during the growth process of a nanotube forest. Alkynes are hydrocarbons which are typically used as feedstocks in manufacturing processes. The CNTs “forests” were grown on a 0.5 × 0.5 cm2 silicon substrate, reaching their terminal heights of over 0.40 millimeters in less than 5 minutes.
The researchers directly incorporated three alkyne types into the CNT during lattice growth—acetylene, methyl acetylene, and vinyl acetylene—with each exhibiting a distinct influence on the final nanotube structure. For example, switching the alkyne precursors from acetylene to methyl acetylene resulted in a real-time decrease in the alignment of the nanotube forest.
This in situ, gas-directed functionalization and atomic-scale manipulation has the potential to enable routes to a variety of previously inaccessible materials and has important implications for understanding how CNTs form on the molecular scale. Such information could be leveraged to create more chemically and structurally complex CNT structures, enable more sustainable chemical pathways by avoiding the need for solvents and postreaction modifications, and potentially unlock experimental routes to a host of higher-order carbonaceous nanomaterials. Future research calls for the scalability and reproducibility of these results.
This project was supported by LLNL’s Laboratory Directed Research and Development (LDRD) Program under project 21-ERD-024 and from the Defense Threat Reduction Agency (DTRA-CB) via grant BA12PHM123.
[M.J. Giannetto, E.P. Johnson, A. Watson, E. Dimitrov, A. Kurth, W. Shi, F. Fornasiero, E.R. Meshot, D.L. Plata, Modifying molecular structure of carbon nanotubes through gas‐phase reactants, ACS Nanoscience Au (2023), DOI: 10.1021/acsnanoscienceau.2c00052.]
–Physical and Life Sciences Communications Team
Tags
Bioscience and BioengineeringBiosciences and Biotechnology
Physical and Life Sciences
Featured Articles