LAB REPORT
Science and Technology Making Headlines
Nov. 12, 2021


An experiment at the National Ignition Facility put researchers at the threshold of fusion ignition, achieving a yield of more than 1.3 megajoules — an eight times improvement over experiments conducted in spring 2021 and a 25 times increase over NIF’s 2018 record yield. Credit: John Jett, LLNL.
On the verge of ignition
Lawrence Livermore nuclear scientists generated 1.3 megajoules (MJ) of energy for the first time with lab-based fusion reactions using lasers the size of three football fields, potentially cementing the ground for a new clean energy source and a better technique to assess the increasing proximity to “greater than unity” levels of energy generation.
The experiment took place at the National Ignition Facility. Experts centered a massive array of nearly 200 laser beams on a tiny spot to create a mega blast of energy — eight times more than they had previously done. Although the energy only lasted 100 trillionths of a second, it moved scientists closer to fusion ignition, the point at which they produce more energy than they consume.
“This result is a historic advance for inertial confinement fusion research,” said Kim Budil, LLNL director.


A multidisciplinary team including an LLNL mathematician has discovered a machine learning-based technique capable of automatically deriving a mathematical model for the motion of binary black holes from raw gravitational wave data.
Black holes make waves
The announcement that the Laser Interferometer Gravitational-wave Observatory (LIGO) had detected gravitational waves during the merger of two black holes sent ripples throughout the scientific community in 2016. The earthshaking news not only confirmed one of Albert Einstein's key predictions in his general theory of relativity, but also opened a door to a better understanding of the motion of black holes and other spacetime-warping phenomena.
Cataclysmic events such as the collision of black holes or neutron stars produce the largest gravitational waves. Binary black holes orbit around each other for billions of years before eventually colliding to form a single massive black hole. During the final moments as they merge, their mass is converted to a gigantic burst of energy — per Einstein's equation E=mc2 — which can then be detected in the form of gravitational waves.
To understand the motion of binary black holes, researchers have traditionally simplified Einstein's field equations and solved them to calculate the emitted gravitational waves. The approach is complex and requires expensive, time-consuming simulations on supercomputers or approximation techniques that can lead to errors or break down when applied to more complicated black hole systems.
Along with collaborators at the University of Massachusetts, Dartmouth and the University of Mississippi, a Lawrence Livermore mathematician has discovered an inverse approach to the problem, a machine learning-based technique capable of automatically deriving a mathematical model for the motion of binary black holes from raw gravitational wave data, requiring only the computing power of a laptop.


Flames consume a home as the Dixie Fire (California’s largest wildfire) tears through the Indian Falls community in Plumas County.
Climate change drives wildfires
As it turns out, human-caused climate change is intensifying the frequency of wildfires. Researchers hadn't expected human-caused global warming to take over from natural climate variability as the main contributor to fire weather until much later this century, around 2080, according to new research by Lawrence Livermore and UCLA.
For the first 20 years of the 21st century, the greenhouse-gas-induced fire weather has already surpassed natural variability. Wildfires razed an average of 5,200 square miles of land per year in the Western U.S. from 2001 to 2018 — nearly double the area burned when compared with the period from 1984 to 2000.
In the new study, scientists found global warming was 68 percent likely the driving factor for atmospheric conditions fueling increasingly destructive wildfires.


Disordered boron surface structure of magnesium diboride probed by atomistic modeling.
Out of order
Lawrence Livermore National Laboratory (LLNL) scientists have found that atomic disorder in certain boron-based hydrogen storage systems can potentially improve the rate of hydrogen uptake.
Metal boride surfaces and their single-layer variants — known as borophenes — are generally thought to feature a regular arrangement of atoms at low to moderate temperatures. The LLNL team proved that in many cases, these atoms actually disorder dynamically: A surprising phenomenon in contrast to conventional understanding of how most solid-state surfaces behave. The surface disorder means each atomic site has different local properties. According to the team’s research, some of these sites can make dissociation of hydrogen molecules easier, which in turn is expected to accelerate activation of the material during hydrogen storage.
The findings have implications for other applications, too. In addition to hydrogen storage, metal borides and borophenes can be used for superconductivity, electrocatalysis, optoelectronics and as coatings for thermal and corrosion resistance.

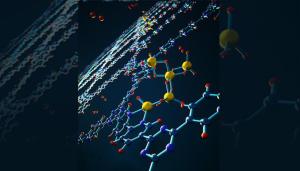
View of a subnanoscale reversible alane cluster coordinated to a bipyridine site on covalent triazine-based framework that can be used in hydrogen storage systems.
New hydrogen storage material is a gas
A team of scientists from Lawrence Livermore National Laboratory and Sandia National Laboratories has proposed to use aluminum hydride (AlH3) to store hydrogen. This solid-state metal hydride, which also is known as “alane,” is commonly used for rocket fuel, explosives, as a reducing agent in alkali batteries, and as a hydrogen source for low-temperature fuel cells.
According to the researchers, this material can overcome the challenge represented by the thermodynamic limitation of hydrides in storing hydrogen. “Many high-capacity metal hydrides suffer from poor thermodynamics of hydrogen uptake after initial release, which necessitates extreme hydrogen pressures to regenerate,” the scientists explained. “Such a limitation is often tied to their metastable nature and hinders their real-world applications.”
At room temperature, aluminum hydride is a metastable crystalline solid and when it goes through an endothermic reaction it decomposes to generate aluminum and hydrogen gas. The research team developed a nanoconfined material with improved thermodynamics and placed it within the nanopores of a highly porous bipyridine-functionalized covalent triazine framework.