LAB REPORT
Science and Technology Making Headlines
Aug. 7, 2020


A new carbon-absorbing material can be extruded (left) or 3-D printed (right) into structures, providing a cheap and efficient way to purify biogas.
Stepping on the gas
Biogas released from decomposing organic matter is about 60 percent methane, making it a valuable renewable source of natural gas, but carbon dioxide, which makes up the other 40 percent, must be removed before the biogas can be turned into fuel. Many of the existing methods for removing the CO2 and other impurities are too expensive or impractical for small-scale biogas sources, like manure digesters on dairy farms.
Lawrence Livermore researchers have addressed this need by developing a new low-cost sorbent made from two familiar compounds: sodium carbonate (also known as washing soda) and silicone.
Although biogas is already being produced as a waste product of industrial and agricultural processes, few of the thousands of small-scale operations in the U.S. are able to invest in turning this waste into renewable fuel. Other CO2-removal methods such as water scrubbing and cryogenic separation are too expensive for these smaller biogas sources. Sarah Baker and her group at LLNL sought to fill that gap.
About five years ago, Baker and her colleagues reported that microcapsules of a sodium carbonate solution encapsulated by silicone — a highly gas-permeable material — could absorb CO2 very efficiently, as the CO2 reacts with the basic sodium carbonate solution to form bicarbonate.


A new model of rotational diamond anvil cell experiments predicts that compressive shearing forces exerted by the tidal pull of Jovian planets on moons like Europa and Enceladus may form a natural reactor for prebiotic chemistry in their rocky ice-covered crusts. Image by Veronica Chen/LLNL.
A fact of life
Massive compressive shearing forces generated by the tidal pull of Jupiter-like planets on their rocky ice-covered moons may form a natural reactor that drives simple amino acids to polymerize into larger compounds. These extreme mechanical forces strongly enhance molecule condensation reactions, opening a new arena of possibilities for the chemical origins of life on Earth and other rocky planets.
That is the conclusion of a new study by Lawrence Livermore National Laboratory (LLNL) scientists who explored the hypothesis that compressive shearing may have driven prebiotic chemistry.
"Compressive shearing forces are known to accelerate physical and chemical transformations in solid materials," said LLNL chemist Brad Steele, lead author of the study, "but little is known about how these processes occur, especially for simple prebiotic molecules like amino acids, which can have a propensity to link together."


New Site-Wide Environmental Impact Statement for LLNL
The Department of Energy’s National Nuclear Security Administration (DOE/NNSA) has approved a Notice of Intent (NOI) to prepare a new Sitewide Environmental Impact Statement for Continued Operation of Lawrence Livermore National Laboratory (LLNL SWEIS). The NOI was published in the Federal Register Aug. 5.
The LLNL SWEIS will analyze the potential environmental impacts of the reasonable alternatives for continuing LLNL operations for approximately the next 15 years.
The SWEIS will analyze at least two alternatives, the No Action Alternative and the Proposed Action. The No Action Alternative, which provides a benchmark for comparison with the environmental effects of the other alternatives, is to continue current LLNL operations of programs in support of assigned missions, without foreseeable new operations and facilities for the next 15 years. The Proposed Action would address aging infrastructure concerns and includes those projects, activities and facilities described for the No Action Alternative, as well as the construction of new facilities, modification of existing facilities, operational changes, and decontamination and decommissioning of excess facilities.
The NOI invites public comment on the scope and alternatives of this SWEIS. In light of the current COVID-19 pandemic, a virtual scoping meeting will be held in place of in-person meetings. Information about how to participate in the virtual meeting and submit comments will be posted on the NNSA NEPA Reading Room web page and also will be announced in local newspapers.

A schematic illustration of a 3D nanometer-thin membrane for ultra-fast selective mass transport. Illustration by Tongshuai Wang/University of Illinois.
Biological cue call
Mimicking the structure of the kidney, a team of scientists from Lawrence Livermore National Laboratory (LLNL) and the University of Illinois at Chicago (UIC) have created a 3D nanometer (nm)-thin membrane that breaks the permeance-selectivity trade-off of artificial membranes.
Highly permeable and selective membranes are useful for a wide range of applications, such as dialysis, water purification and energy storage. However, conventional synthetic membranes based on two-dimensional structures suffer from the trade-off limitation between permeability and selectivity, arising from their intrinsically limited surface area and long complex pore geometries.
Taking a cue from biological systems that achieve a highly selective and rapid trans-membrane mass transport by employing efficient 3D functional structures, the team developed a self-supportive 3D membrane composed of two 3D interconnected channels, which are separated by a nanometer-thin porous titanium-oxide (TiO2) layer.

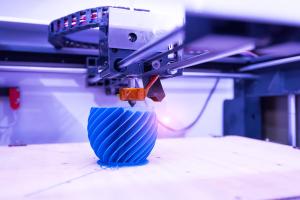
LLNL scientists and collaborators are using machine learning to address two key barriers to industrialization of two-photon lithography: monitoring of part quality during printing and determining the right light dosage for a given material.
Practice makes perfect
Two-photon lithography (TPL) — a widely used 3D nanoprinting technique that uses laser light to create 3D objects — has shown promise in research applications but has yet to achieve widespread industry acceptance due to limitations on large-scale part production and time-intensive setup.
Capable of printing nanoscale features at a very high resolution, TPL uses a laser beam to build parts, focusing an intense beam of light on a precise spot within a liquid photopolymer material. The volumetric pixels, or "voxels," harden the liquid to a solid at each point the beam hits and the uncured liquid is removed, leaving behind a 3D structure. Building a high-quality part with the technique requires walking a fine line: too little light and a part can't form, too much and it causes damage. For operators and engineers, determining the correct light dosage can be a laborious manual process.
Lawrence Livermore scientists and collaborators turned to machine learning to address two key barriers to industrialization of TPL: monitoring of part quality during printing and determining the right light dosage for a given material. The team's machine learning algorithm was trained on thousands of video images of builds labeled as "uncured," "cured," and "damaged," to identify the optimal parameters for settings such as exposure and laser intensity and to automatically detect part quality at high accuracy.

This illustration depicts lanmodulin, a small protein which is a bio-sourced alternative to extract, purify and recycle rare earth elements from various sources, including electronic waste. Lanmodulin is the most selective macromolecule for rare earth elements characterized to date and may offer a new paradigm for extractive metallurgy and other applications. Illustration by Thomas Reason/LLNL.
In rare form
Rare earth elements (REEs) are critical to making lasers and certain magnets, fluorescent lamps and sonar systems, computer memories and X-ray tubes.
There are 17 REEs, and most of them play a special role in 21st century living — several pounds of these compounds, for instance, are used in batteries for electric and hybrid vehicles.
Ironically, most REEs are not particularly rare. Rather, they are seldom found in concentrated amounts large enough to make them readily profitable to extract and refine.
In research funded by the Department of Energy, Lawrence Livermore National Laboratory (LLNL), and collaborators are jointly developing a new protein-based environment-friendly process to extract and purify REEs from low-grade sources, which otherwise require toxic chemicals to process. The bio-sourced compound known as lanmodulin “has an unprecedented appetite and selectivity for REEs,” said Gauthier Deblonde, a staff scientist at LLNL. “And our collaboration has yielded a completely new and green process for REE extraction and purification.”
To date, the researchers have tested the process using electronic waste containing a broad range of impurities and now believe it will work with all 17 REEs. “Many alternative secondary sources containing REEs have remained untapped because there is no efficient method to extract them,” Deblonde said. “Our green lanmodulin-based approach will open up various opportunities to produce or recycle REEs.”





