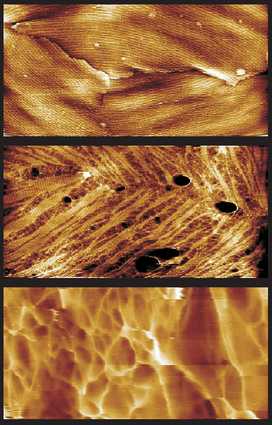
Researchers track how spores break outof dormant state
LIVERMORE, Calif. – Tapping into the unknown world of awakening dormant bacterial spores, researchers have revealed through atomic force microscopy (AFM) the alterations of spore coat and germ cell wall that accompany the transformation from a spore to a vegetative cell.
When starved of nutrients Bacillus (rod-shapedbacteria) cells initiate a series of genetic,biochemical and structural events that resultin the formation of metabolically dormantspores.
They can remain dormant for extended periods and, partly because of their tough spore coat, have a significant resistance to extreme environmental factors including heat, radiation and toxic chemicals. However, once in favorable conditions, spores break the dormant state through germination and reenter the vegetative mode of replication.
Although significant progress has been madein understanding the biochemical and geneticbases of the spore germination process, itis still unclear how a spore breaks out ofits dormant state.
But a new in vitro study of singlegerminating Bacillus atrophaeus sporesdetails how the spore coat structures breakdown, and it shows with unprecedented resolutionhow the new bacterium emerges from the disintegratingspore.
The new research, led by Lawrence Livermore National Laboratory scientists, appears in the June 4 issue of the Proceedings of the National Academy of Sciences .
"A thorough understanding of sporegermination is important for the developmentof new countermeasures that identify theearliest stages of a wide range of sporemediated diseases, including botulism, gasgangrene and pulmonary anthrax," saidAlexander Malkin, senior author from LLNL’sBiosciences and Biotechnology Division.
"Butit’s also important to gain fundamentalinsights into the key events in bacterialcell development."
The researchers, including Marco Plomp, leadauthor at LLNL, and those from Children’sHospital Oakland Research Institute and NorthwesternUniversity, used AFM to identify disassemblyof the outer spore coat rodlet structures,which appear to be structurally similar toamyloid fibrils that have been associatedwith neural degenerative diseases, such asAlzheimer’s and prion diseases.
"Theextreme physical and chemical resistanceof Bacillus spores suggests thatevolutionary forces have captured the mechanicalrigidity and resistance of these amyloidself-assembling biomaterials to structurethe protective outer spore surface," Plompsaid.
When exposed to a solution that triggersgermination, nanometer sized etch pits wereseen developing in the rodlet layer. Theseetch pits evolved into ever widening fissures,leaving narrow strips of remaining rodletstructure. In the end, 1- to 3- nm-wide fibrilsremained. The in vitro AFM imagingalso revealed the porous fibrous cell wallstructure of newly emerging and mature vegetativecells, consisting of a network of nanometer-widepeptidoglycan fibers.
"These resultsshow that dynamic AFM is a promising toolto investigate the formation and evolutionof the bacterial cell wall," Malkinsaid.
The research is funded by LLNL’s LaboratoryDirected Research and Development program,the Defense Advanced Research Project Agency(DARPA) and the Federal Bureau of Investigation.
Founded in 1952, Lawrence Livermore National Laboratory is a national security laboratory, with a mission to ensure national security and apply science and technology to the important issues of our time. Lawrence Livermore National Laboratory is managed by the University of California for the U.S. Department of Energy’s National Nuclear Security Administration.
Contact
Anne M. Stark[email protected]
925-422-9799
Related Links
In vitro high-resolution structural dynamics of single germinating bacterial sporesThe high-resolution architecture and structural dynamics of Bacillus spores
Architecture and high-resolution structure of Bacillus thuringiensis and Bacillus cereus spore coat surfaces