New look at cell membrane reveals surprising organization
 (Download Image)
(Download Image)
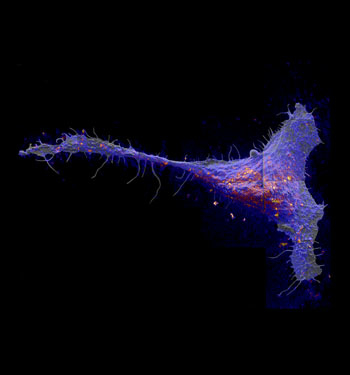
The local abundance of metabolically incorporated 15N-sphingolipids in the plasma membrane of a fibroblast cell, overlaid on the corresponding secondary electron image. Red and yellow colors are local elevations in sphingolipid abundance.
A novel imaging study by researchers from Lawrence Livermore National Laboratory, the University of Illinois and the National Institutes of Health revealed some unexpected relationships among molecules within cell membranes.
Their findings provide a new way of studying cell structure and ultimately its function.
Led by Mary Kraft of the University of Illinois, Peter Weber of Lawrence Livermore National Laboratory and Joshua Zimmerberg of the National Institutes of Health, the team published their findings in the online version of the Jan. 28 edition of the Proceedings of the National Academy of Sciences.
Cells are enveloped in a semi-permeable membrane that acts as a barrier between the inside and outside of the cell. The membrane is mainly composed of a class of molecules called lipids, which are small and easily perturbed when tracked.
"Lipids have multiple functions serving as both membrane structure and signaling molecules, so they regulate other functions inside the cell," Kraft said. "Therefore, understanding how they're organized is important. You need to know where they are to figure out how they're performing these regulatory functions."
Previous cell membrane research suggested that lipids in the membrane assemble into patches, called domains, which differ in composition. But the challenge of direct observation has limited research into how lipids are organized in the membrane, and how that organization affects cell function. In the new study, the team used an advanced, molecule-specific imaging method developed at Lawrence Livermore that allowed the researchers to look at the membrane itself and map a particular type of lipid on mouse cell membranes. The researchers at University of Illinois fed lipids labeled with rare stable isotopes to the cells and then imaged the distribution of the isotopes with high-resolution imaging mass spectrometry at LLNL.
Called sphingolipids (sfing-go-lipids), these molecules are thought to associate with cholesterol to form small domains about 200 nanometers across. The direct imaging method revealed that sphingolipids do form domains, but not in the way the researchers expected.
The domains were much bigger than results from prior experiments. The 200-nanometer domains clustered together to form much larger, micrometer-sized patches of sphingolipids in the membrane.
"We were amazed when we saw the first images of the patches of sphingolipids across the cell surface," said Peter Weber, who directed the team at Lawrence Livermore. "At the start, we weren't sure if our imaging mass spectrometry method would be sensitive enough to detect the labeled lipids, let alone what we would see."
When the researchers looked at cells that were low on cholesterol -- thought to play a key role in lipid aggregation - they were surprised to find that the lipids still formed domains. But disruption to the cell's structural scaffold seemed to dissolve the lipid clusters. "We found that the presence of domains was somewhat affected by cholesterol but was more affected by the cytoskeleton -- the protein network underneath the membrane," Kraft said. "The central issue is that the data is suggesting that the mechanism that's responsible for these domains is much more complicated than initially expected."
In addition, the new study found that sphingolipid domains were incompletely associated with a marker protein that researchers have long assumed lived where sphingolipids congregated. This means that data collected with imaging techniques that target this protein are not as accurate in representing sphingolipid distribution as previously thought.
"Our data is showing that if you want to know where sphingolipids are, look at the lipid, don't infer where it is based on other molecules," Kraft said, "and now there's a way to directly image them."
The researchers plan to use the direct-imaging method in conjunction with other more conventional methods, such as fluorescence, to further determine the organization of different kinds of molecules in the membrane, their interactions and how they affect the cell's function. They plan to begin by targeting cholesterol.
"Cholesterol abundance is important. You change that, you tremendously change cell function," Kraft said.
Other Livermore researchers include Ian Hutcheon and Kevin Carpenter.
The LLNL Laboratory Directed Research and Development program, National Institutes of Health, the National Science Foundation and the Burroughs Wellcome Fund supported this work. Co-author Joshua Zimmerberg directed research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, part of the National Institutes of Health.
Contact
Anne M Stark[email protected]
925-422-9799
Related Links
Peter WeberResearch advances understanding of how cell membranes function
Piecing together the cyanobacteria puzzle
Tags
Physical and Life SciencesFeatured Articles