Lab team sets sight on new artificial retina
Laboratory researchers are now developing the implantable system for a third-generation artificial retina as part of a U.S. Department of Energy (DOE) project to produce an "retinal prosthesis" that could restore vision to millions of people suffering from eye diseases.
R&D 100 Magazine recently announced that the second generation artificial retina, Argus II, has been selected for a 2009 R&D 100 award. R&D Magazine issues the R&D 100 Awards every year for the most innovative technologies in science.
The DOE artificial retina project brings together five national labs, four universities and a private company, and Lawrence Livermore now serving as the lead organization for technical work on the project.
Researchers at the Lab are today using advanced polymer-based micro-fabrication methods to further develop a biocompatible microelectrode array for the third-generation artificial retina device.
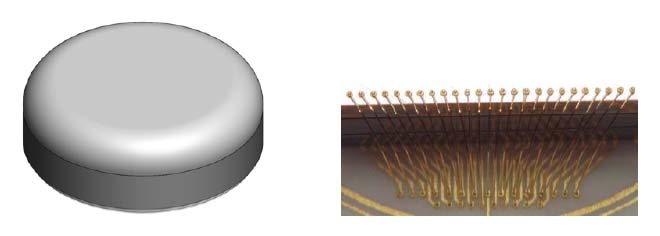
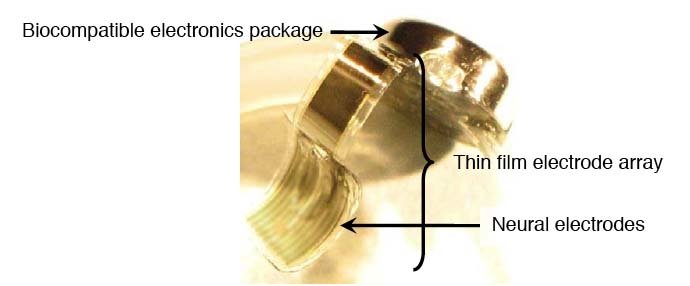
The LLNL team contributes three major components to the artificial retina: the thin-film electrode array that contains the neural electrodes; the biocompatible electronics package that contains the electronics for stimulating the retina and wireless power and communications; and an ocular surgical tool that will enable the replacement of the thin-film electrode array. In addition, Lawrence Livermore is responsible for the system integration and assembly of the complete implantable artificial retina system.
"This project is a fine example of what multi-disciplinary, multi-institutional science teams can accomplish to address a major global health problem," said principal investigator Satinderpall Pannu, leader of the Livermore group. "We're very proud of the expanded role we at Livermore are playing in the development of this retinal prosthesis, which has the potential to improve the quality of life for so many people around the world."
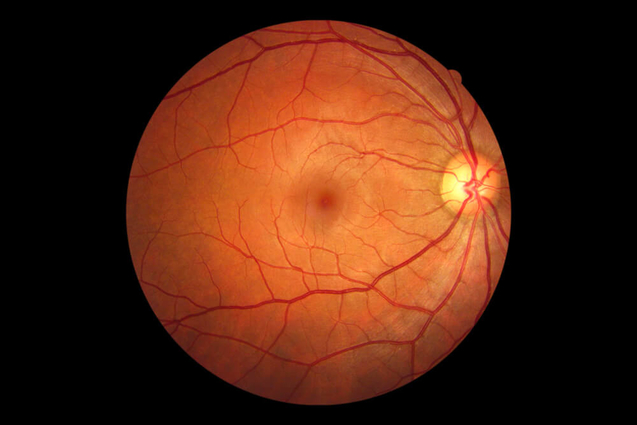
An artificial retina or "retinal prosthesis" has the potential to restore vision to millions of people suffering from eye diseases such as retinitis pigmentosa , macular degeneration or those who are legally blind due to the loss of photoreceptor function. In many cases, the neural cells to which the photoreceptors are connected remain functional. Project leader Dr. Mark Humayun, of the University of Southern California's Doheny Eye Institute, demonstrated that electrical stimulation of the viable retinal cells can result in visual perception. These findings have sparked a worldwide effort to develop a retinal prosthesis device.
The R&D 100 award has been given to the second-generation retinal prosthesis, which has been implanted in some 29 patients in the United States and Europe as part of clinical trials. The second-generation device represents a substantial vision and performance improvements over the first-generation device in speed of recognition and resolution. Objects can now be recognized within two to three seconds, and the device's 60 electrodes have improved image resolution over the 16-electrode prosthesis.
The prosthesis is now of sufficient resolution to allow recognition of doors, windows, edges, low-lying branches and the square on a basketball backboard. The goal of the DOE project is to produce a prosthesis with more than 1,000 electrodes, which would allow facial recognition.
Expertise in biomedical microsystems at Lawrence Livermore's Center for Microtechnology was tapped in 2001 to develop a "flexible thin film microelectrode array" able to conform to the curved shape of the retina, without damaging the delicate retinal tissue, and to integrate electronics developed by University of California at Santa Cruz. The device serves as the interface between an electronic imaging system and the human eye, directly stimulating neurons via thin film conducting traces and electroplated electrodes.
The Lab's artificial retina team led by Pannu includes Phillipe Tabada, Courtney Davidson, Terri Delima, Julie Hamilton, William Benett, Erika Fong, Emil Geiger, Maxim Shusteff, and Kedar Shah. Pannu's group is part of the Lab's Center for Micro-and Nano-Technology and applies its expertise to develop microelectromechanical systems, called "MEMS." These systems are the result of integrating micrometer-sized mechanical elements, sensors, actuators and electronics through microfabrication technology.
The metal traces that form the electrodes in the array and the piece of the prosthesis that is placed on the retina, are less than 10 micrometers thick — less than 10 percent of the thickness of a human hair. The electrode array is embedded in a soft biocompatible polymerto allow it to conform to the curvature of the retina.
The elecrtronics in the prosthesis stimulate the retina by sending electrical signals to microelectrodes, a function normally produced by a healthy eye's photoreceptor cells. In people suffering blindness because of age-related macular degeneration or retinitis pigmentosa this process has broken down. The prosthesis' microelectrode array performs the function of the photoreceptor cells by electrically stimulating remaining healthy ganglion cell layers.
The "visual" signal is transmitted to the implant device from a mini-camera mounted on a pair of dark glasses. A battery pack worn on a belt powers the system and offers some controls for adjusting the device.
The challenge for Pannu's group is to further reduce the size and complexity of the retinal prosthesis and greatly increase the number of electrodes that provide greater resolution and, consequently, even clearer vision.
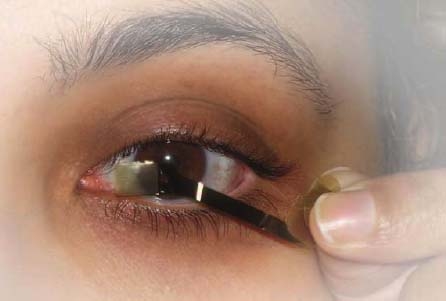
In addition, the Lab is using its expertise in microdevices to develop an advanced ocular surgical tool that allows surgeons to implant the microelectrode array with great precision and minimal tissue damage. The system developed by Lab engineers allows surgeons to remove an array and replace it with an updated version.
"This is a motivating project to work on," Pannu said, noting the often plaintive e-mails and letters he has received from blind people all over the world who have read about his work with the DOE project and offer themselves as subjects for clinical trials. "They're looking for a ray of hope."
Project background
The artificial retina team was established through a Department of Energy-sponsored Cooperative Research and Development Agreement (CRADA) in 2004 with the mission of developing the world's most advanced high-density microelectronic–tissue hybrid prosthesis for imaging.
The five national laboratories are: Argonne, Lawrence Livermore, Los Alamos, Oak Ridge, and Sandia national laboratories. The four universities are USC (Doheny Eye Institute), California Institute of Technology, North Carolina State University and the UC Santa Cruz; and the industrial partner is Second Sight® Medical Products Inc., the group responsible for commercializing the product and conducting clinical trials.
For more information about the project, check related Websites: