LAB REPORT
Science and Technology Making Headlines
Sept. 14, 2018

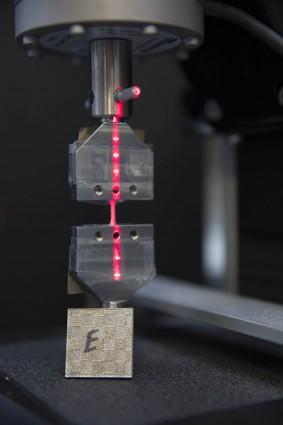
The latest High Performance Computing for Manufacturing (HPC4Mfg) solicitation calls for industry projects focused on improving U.S. steel and aluminum manufacturing.
Calling all steel manufacturers
The Department of Energy (DOE), in partnership with Lawrence Livermore National Laboratory, wants American steel and aluminum manufacturers to inquire within.
DOE recently announced $1.2 million for four new projects to support American steel and aluminum manufacturers in improving energy efficiency, increasing productivity and accelerating manufacturing innovation.
The funding will provide advanced technology to improve manufacturing processes and resolve key manufacturing challenges through the use of high performance computing. This special call for concept papers marks the sixth solicitation for the High Performance Computing for Manufacturing (HPC4Mfg) program.
Selected demonstration projects will be awarded up to $300,000 to support compute cycles and work performed by the national lab partners. Industry partners must provide a participant contribution of at least 20 percent of the DOE funding for the project.


Jupiter is not only the largest planet in our solar system, it’s also the oldest, according to research from Lawrence Livermore National Laboratory. Credit: NASA.
Better late than never
New research suggests why Jupiter, the largest planet in the solar system, waited about 2 million years for its early formation growth spurt.
A team led by a Swiss researcher found that kilometer-size worlds smashed into the giant planet during that time, generating zones of high energy. This bombardment made it difficult for gas molecules to accrete, forcing the planet to grow more slowly.
The solar system is about 4.5 billion years old, and the popular formation theory for planets says that they were formed out of an orbiting cloud of gas and dust that surrounded the young sun. Over time, the gas and dust clumped into small worlds, gradually accreting to each other to form the planets.
This new data lined up with research by Thomas Kruijer, a researcher at Lawrence Livermore National Laboratory, who conducted research on observations of meteorite compositions.
Kruijer’s team found that the meteorites studied appeared to come from two “reservoirs” in our solar system that were separated into the inner zone and the outer zone, starting about 1 million years after the solar system formed. The pull of Jupiter, the researchers said, was the reason that material from the outer solar system, where Jupiter and the gas giants reside, could not interact with the inner part of the solar system, where Earth and other rocky planets orbit today.

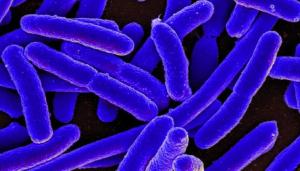
A colorized scanning electron micrograph of Escherichia coli (E. coli). LLNL researchers and collaborators have developed a new system to control gene expression in laboratory bacteria. Credit: National Institute of Allergy and Infectious Diseases, National Institutes of Health
All aboard the ‘Jungle Express’
The microbial production of enzymes, chemicals and fuels could become more efficient and economical with a newly engineered system for controlling genes called "Jungle Express."
Scientists and collaborators from the Joint BioEnergy Institute, Lawrence Livermore National Laboratory (LLNL), Lawrence Berkeley National Lab and San Francisco State University have developed a new system to control gene expression in laboratory bacteria.
Bacteria can serve as living factories to produce a wide array of biochemical products. But many bacterial large-scale fermentations have not been efficient before because of the lack of affordable genetic control elements.
“These enhancements to bacterial gene regulation have a potential translation to sustainable bioproduction," said LLNL biochemist Michael Thelen.
Both in fundamental research and at industrial scale, it is often necessary to maintain strict control over the expression of a gene or set of genes that have been placed into bacterial cells for the biosynthesis of target products.

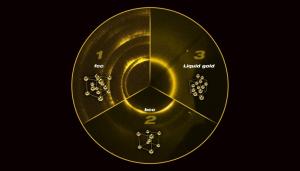
Combining 3D printing with an alloying and dealloying process, researchers at Lawrence Livermore and Harvard were able to engineer nanoporous gold into microarchitectured hierarchical structures, a development that revolutionize the design of chemical reactors. Credit: Ryan Chen/LLNL
Good as gold
Lawrence Livermore National Laboratory (LLNL) is known for doing impressive work with materials, particularly related to 3D printing. Whether it’s nanoscale 3D printing or 3D-printed glass, the organization is making new discoveries.
In its latest study, LLNL researchers along with those from Harvard University discuss the hierarchical printing of nanoporous gold. According to the researchers, this work could have a major impact on the design of chemical reactors.
Nanoporous metals are strong catalysts for chemical reactions, as they have a large surface area and high electrical conductivity. This makes them well-suited to applications such as electrochemical reactors, sensors and actuators.
The researchers combined an extrusion-based direct ink writing process with an alloying and dealloying process to engineer nanoporous gold into three distinct scales, from the microscale to the nanoscale.


A sample of microarchitectured graphene aerogel, made from one of the lightest materials on Earth, sits atop a flower.
Graphene is going strong
With its incredible strength and potentially miraculous applications, there is a lot to be enthusiastic about when it comes to graphene. But it’s one thing to show off these possibilities in a lab; another to turn it into something that’s usable in real-world situations.
That’s something that researchers from Lawrence Livermore National Laboratory and Virginia Tech have been working to change. In the process, they have found a way of combining two of the most promising buzzwords in tech — “graphene” and “3D printing” — to open up new possibilities.
Regular graphene is a single layer of carbon atoms arranged in a honeycomb-style hexagonal lattice pattern. If graphene is packed, layer on layer, it becomes graphite: A material most commonly used as the “lead” in ordinary pencils. Anyone who has ever had a pencil lead snap on them may have a hard time believing this is one of the strongest materials on the planet. That’s because of the way that it is packed together, which fundamentally alters the structure of graphene.
The researchers on this project circumvented that by separating the individual sheets of graphene with air-filled pores, thereby allowing it to maintain its properties. The 3D-printable material that emerges at the end is graphene aerogel.





