LAB REPORT
Science and Technology Making Headlines
Oct. 15, 2021


A NASA flyby of the asteroid Apophis, which is in the category of Potentially Hazardous Asteroids (PHAs) - asteroids with orbits that bring them very close to Earth now and for centuries in the future.
Pushing collision-course asteroids out of the way
Launching a nuclear bomb at an asteroid on collision course with Earth may sound like the plot of a sci-fi movie, but new research suggests even a terrifyingly late intervention could help avoid an extinction-level crash. The potential for a so-called asteroid disruption has been well argued over recent decades, as astronomers and defense agencies try to figure out just what Earth could do in the fact of an asteroid strike.
Efforts for long-distance detection of potentially dangerous asteroids that might one day present a risk to Earth is still underway. Unfortunately, there’s always the possibility of a rogue rock slipping through the net. Lawrence Livermore National Laboratory (LLNL) researchers looked at just what might be involved for a late-stage intervention through either kinetic impactor or nuclear explosion means.
LLNL’s Planetary Defense Group calculated the varying impact of different sizes of intervention carried out at different timescales. While scientists would prefer to have more warning time, they need to be prepared for any possible scenario, as many near-Earth asteroids remain undiscovered.

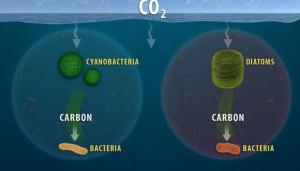
The study investigates how marine bacteria are adapted to consume the carbon produced by the two major types of photosynthetic phytoplankton in the oceans (diatoms and cyanobacteria), which has implications for our understanding of carbon sequestration in the current and future ocean. Image by Michelle Zatcoff.
It’s something in the water
Marine-dissolved organic matter, which originates from phytoplankton, holds as much carbon as Earth’s atmosphere, yet the biological processes governing its fate are primarily studied under idealized laboratory conditions or through indirect measures such as genome sequencing.
In new research by a Lawrence Livermore National Laboratory (LLNL) scientist and collaborators at Oregon State University and Oak Ridge National Laboratory, the team used a relatively new technique to directly quantify uptake of complex carbon pools from the two primary sources of marine organic carbon (diatoms and cyanobacteria) by a natural microbial community.
The research is an important step toward ultimately forecasting how much carbon will leave the ocean and wind up in the atmosphere and how much will end up entombed in marine sediments.
“We provide functional insights into the activity of microbes during marine phytoplankton blooms,” said Xavier Mayali, LLNL scientist and a co-author of the study.


Rare-earth metals are an essential component of mobile phones, computers and many other everyday devices
A rare find
Lawrence Livermore researchers and collaborators have found a a new method to improve the extraction and separation of rare earth elements — a group of 17 elements critical for technologies such as smart phones and electric car batteries — from unconventional sources.
New research by LLNL and Penn State demonstrates how a protein isolated from bacteria can provide a more environmentally friendly way to extract these metals and to separate them from other metals and from each other. The method could eventually be scaled up to help develop a domestic supply of rare earth metals from industrial waste and electronics due to be recycled.
Because the U.S. currently imports most of the rare earth elements it needs, a new focus has been placed on establishing a domestic supply from unconventional sources, including industrial waste from burning coal and mining other metals as well as electronic waste from cell phones and many other materials. These sources are vast but considered “low grade,” because the rare earths are mixed with many other metals and the amount of rare earths present is too low for traditional processes to work well. Furthermore, current methods for extraction and separation rely on harsh chemicals, are labor intensive, sometimes involve hundreds of steps, produce a high volume of waste and are expensive.
The new method takes advantage of a bacterial protein called lanmodulin, previously discovered by the research team, that is almost a billion times better at binding to rare earth elements than to other metals.


Ice VII is a cubic crystal phase that can form at high pressure and high temperature. It is thought that this “hot ice” could be found on the ocean floor of Saturn's moon Titan and other watery exoplanets.
So hot it’s cool
Water can exhibit some strange behavior when compressed or cooled rapidly. New research involving Lawrence Livermore scientists shows that water can remain liquid in a metastable state when transitioning from liquid to a dense form of ice at higher pressures than previously measured.
Water at extreme conditions has attracted recent attention because of its complex phase diagram, including superionic ice phases having exotic properties that exist at high pressures and densities. To date, 20 unique crystalline ice phases have been found naturally on Earth or in the laboratory. Water also exhibits bizarre metastable phenomena when compressed or cooled very rapidly, which have attracted interest from physicists worldwide for many years.
“If the water is compressed very rapidly, it will remain liquid in a metastable state until finally crystallizing into ice VII at a higher pressure than expected,” said Michelle Marshall, a research scientist at the Laboratory for Laser Energetics at the University of Rochester and a former LLNL postdoc.
Ice VII is the stable polymorph of water at room temperature and at pressures exceeding ∼2 GPa (more than 19,000 atmospheres]. Recently, ice VII was found naturally on Earth for the first time as inclusions in diamonds sourced deep within the mantle. This “hot ice” could be found inside Jupiter’s icy moons and in water worlds beyond our solar system.


A piece of carbonaceous chondrite that contains a large calcium-aluminum-rich inclusion similar to those used in this study. Photo by: Quinn Shollenberger/LLNL.
Born and bred in a place far, far away
The earliest solids formed in the solar system give clues to what radioactive species were made by the young sun, and which ones were inherited.
By studying isotopic variations of the elements vanadium (V) and strontium (Sr), an international team of researchers including scientists from Lawrence Livermore National Laboratory (LLNL) found that those variations are not caused by irradiation from the sun but are produced by condensation and evaporation reactions in the early solar system, according to a recent study.
“It turns out that some of the short-lived radioactive isotopes researchers previously thought were products of irradiation from the early active sun are instead most likely inherited from our parent molecular cloud, which, in turn, tells us a significant amount about the cosmic neighborhood we grew up in,” said LLNL cosmochemist Greg Brennecka.
Calcium-Aluminum-rich inclusions (CAIs) in meteorites are the oldest dated solids that formed within the solar system. They carry crucial information regarding the environmental conditions of the earliest stages of the protoplanetary disk before any of the planets formed. This research also suggests that the oldest solids in our solar system could have formed further away from the sun than previously thought, with far-reaching implications regarding the dynamical structure of the nascent solar system.





