Hydrogen storage reactions reveal a complex dance toward faster uptake
 (Download Image)
(Download Image)
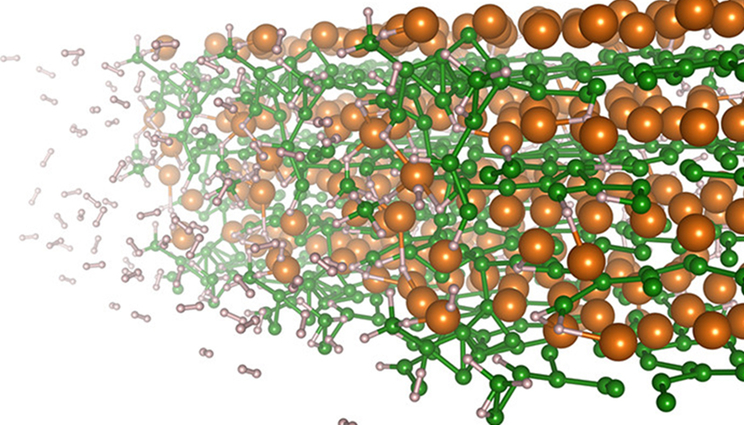
Hydrogen absorption at the surface of magnesium diboride studied with first principles simulations.
Lawrence Livermore National Laboratory (LLNL) scientists have simulated the hydrogen storage reactions in a promising material and discovered why hydrogen uptake slows as the material absorbs hydrogen, providing insight that could be used for improvements.
Improving hydrogen storage in solid-state materials depends on a better understanding of multistep chemical reactions that take place at complex interfaces. At these interfaces, the material transforms from containing no hydrogen to a hydrogen-saturated phase as its constituent molecular units react and bind with hydrogen and structurally rearrange. Analogous transformations govern a variety of chemical and electrochemical energy-storage contexts, from hydrogen storage materials to batteries.
To reveal the underlying mechanisms involved in the hydrogenation of magnesium diboride (MgB2), a team of LLNL scientists have used molecular dynamics simulations. They found that magnesium ions (Mg2+) drive the electric polarization of molecular units and charge redistribution critical for cleaving boron (B) from the original MgB2 material and enabling sequential hydrogen binding to B atoms to form the hydrogen-saturated Mg(BH4)2 phase. Specifically, nearby Mg2+ ions polarize BHX units, allowing the positively charged center boron atom to attract and bind with hydrogen anions, which are negatively charged through interactions with Mg2+. The research appears in the journal Applied Materials & Interfaces.
The analysis additionally revealed a possible explanation for the slowing of hydrogen uptake in MgB2 as Mg(BH4)2 is formed, which prevents full hydrogenation without high temperature and pressure in experiments. Boron contained in the hexagonal sheets of MgB2 is less stable, and therefore more prone to bind hydrogen when the local environment is Mg-poor. However, as the material transforms to Mg(BH4)2, the surfaces of the remaining MgB2 material become more Mg-rich, slowing hydrogenation.
"Our simulations capture the reaction pathways in MgB2 that lead to hydrogen absorption," said LLNL physicist and author Keith Ray. "Hopefully this understanding will enable further research to unlock rapid hydrogenation at lower temperatures and pressures."
Other LLNL authors include ShinYoung Kang, Liwen Wan, Sichi Li, Tae Wook Heo, Jonathan Lee, Alexander Baker and Brandon Wood. The work is funded by the Department of Energy, Office of Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cell Technologies Office, including the Hydrogen Materials Advanced Research Consortium.
Contact
 Anne M. Stark
Anne M. Stark
[email protected]
(925) 422-9799
Related Links
Applied Materials & InterfacesOffice of Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cell Technologies Office
Hydrogen Materials Advanced Research Consortium
Tags
Physical and Life SciencesScience
Featured Articles







