Going inside to get a more efficient catalyst
 (Download Image)
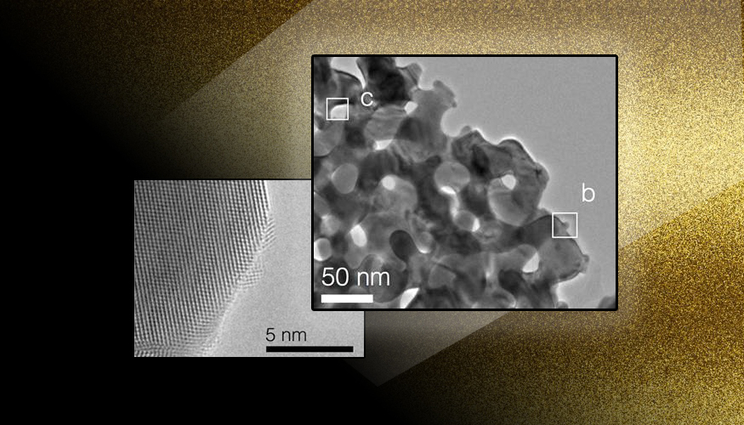
At left is s an aberration-corrected environmental-transmission electron microscopy (TEM) image revealing the formation of highly crystalline metallic nanoparticles during activation of nanoporous gold. At right is a low-magnification TEM image showing the pore and ligament structure of nanoporous gold.
(Download Image)
At left is s an aberration-corrected environmental-transmission electron microscopy (TEM) image revealing the formation of highly crystalline metallic nanoparticles during activation of nanoporous gold. At right is a low-magnification TEM image showing the pore and ligament structure of nanoporous gold.
New research shows that the phases that nano-structured materials go through to become an efficient catalyst are as good as gold.
Lawrence Livermore National Laboratory (LLNL) material scientist Juergen Biener and collaborators found that by restructuring nanoporous gold alloys they become more efficient catalysts.
Nano-structured materials hold promise for improving catalyst activity and selectivity, but little is known about the dynamic compositional and structural changes that these systems undergo during pre-treatment, which leads to efficient catalyst function. (A catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions than otherwise possible.)
The team used ozone-activated silver-gold alloys in the form of nanoporous gold (npAu) as a case study to demonstrate the dynamic behavior of bi-metallic systems during activation to produce a functioning catalyst.
Nanoporous gold, a porous metal, can be used in electrochemical sensors, catalytic platforms, fundamental structure property studies at the nanoscale and tunable drug release. It also features high effective surface area, tunable pore size, well-defined conjugate chemistry, high electrical conductivity and compatibility with traditional fabrication techniques.
"Our results demonstrate that characterization of these dynamic changes is necessary to unlock the full potential of bi-metallic catalytic materials," Biener said.
Advanced in-situ electron microscopy and X-ray photoelectron spectroscopy were used to demonstrate that major restructuring and compositional changes occur along the path to catalytic function.
The research appears in the Dec. 19 edition of the journal, Nature Materials. Other institutions include: Harvard University, Lawrence Berkeley National Laboratory and Brookhaven National Laboratory.
This work was supported as part of the Integrated Mesoscale Architectures for Sustainable Catalysis, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences.
Contact
 Anne M. Stark
Anne M. Stark
[email protected]
(925) 422-9799
Related Links
Nature MaterialsResearchers go for the gold on a single chip
Implantable electrode coating as good as gold
Tags
Physical and Life SciencesFeatured Articles







