Giant lasers crystallize water with shockwaves, revealing the atomic structure of superionic ice
 (Download Image)
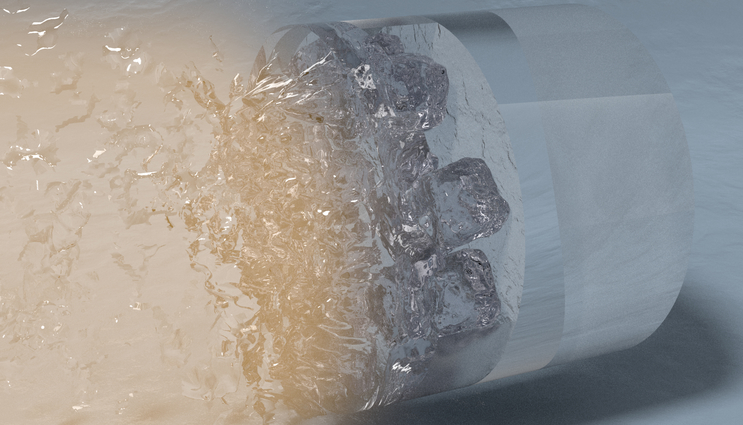
In this artistic rendering of the laser compression experiment, high power lasers focus on the surface of a diamond, generating a sequence of shock waves that propagate throughout the sample assembly (from left to right), simultaneously compressing and heating the initially liquid water sample, forcing it to freeze into the superionic water ice phase. Credit: Millot, Coppari, Hamel, Krauss (LLNL)
(Download Image)
In this artistic rendering of the laser compression experiment, high power lasers focus on the surface of a diamond, generating a sequence of shock waves that propagate throughout the sample assembly (from left to right), simultaneously compressing and heating the initially liquid water sample, forcing it to freeze into the superionic water ice phase. Credit: Millot, Coppari, Hamel, Krauss (LLNL)
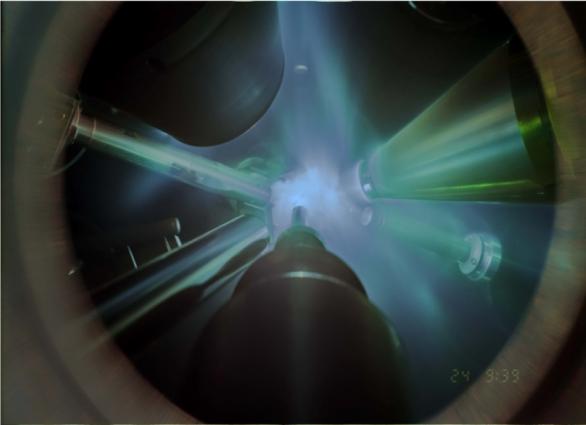
Scientists from Lawrence Livermore National Laboratory (LLNL) used giant lasers to flash-freeze water into its exotic superionic phase and record X-ray diffraction patterns to identify its atomic structure for the very first time — all in just a few billionths of a second. The findings are reported today in Nature.
In 1988, scientists first predicted that water would transition to an exotic state of matter characterized by the coexistence of a solid lattice of oxygen and liquid-like hydrogen — superionic ice — when subjected to the extreme pressures and temperatures that exist in the interior of water-rich giant planets like Uranus and Neptune. These predictions remained in place until 2018, when a team led by scientists from LLNL presented the first experimental evidence for this strange state of water.
Now, the LLNL scientists describe new results. Using laser-driven shockwaves and in-situ X-ray diffraction, they observe the nucleation of a crystalline lattice of oxygen in a few billionths of a second, revealing for the first time the microscopic structure of superionic ice.
The data also provides further insight into the interior structure of ice giant planets.
"We wanted to determine the atomic structure of superionic water," said LLNL physicist Federica Coppari, co-lead author of the paper. "But given the extreme conditions at which this elusive state of matter is predicted to be stable, compressing water to such pressures and temperatures and simultaneously taking snapshots of the atomic structure was an extremely difficult task, which required an innovative experimental design."
The researchers performed a series of experiments at the Omega Laser Facility at the University of Rochester’s Laboratory for Laser Energetics (LLE). They used six giant laser beams to generate a sequence of shockwaves of progressively increasing intensity to compress a thin layer of initially liquid water to extreme pressures (100-400 gigapascals (GPa), or 1-4 million times Earth’s atmospheric pressure) and temperatures (3,000-5,000 degrees Fahrenheit).
"We designed the experiments to compress the water so that it would freeze into solid ice, but it was not certain that the ice crystals would actually form and grow in the few billionths of a second that we can hold the pressure-temperature conditions," said LLNL physicist and co-lead author Marius Millot.
To document the crystallization and identify the atomic structure, the team blasted a tiny iron foil with 16 additional laser pulses to create a hot plasma, which generated a flash of X-rays precisely timed to illuminate the compressed water sample once brought into the predicted stability domain of superionic ice.
"The X-ray diffraction patterns we measured are an unambiguous signature for dense ice crystals forming during the ultrafast shockwave compression demonstrating that nucleation of solid ice from liquid water is fast enough to be observed in the nanosecond timescale of the experiment," Coppari said.
"In the previous work we could only measure macroscopic properties such as internal energy and temperature," Millot added. "Therefore, we designed a new and different experiment to document the atomic structure. Finding direct evidence for the existence of crystalline lattice of oxygen brings the last missing piece to the puzzle regarding the existence of superionic water ice. This gives additional strength to the evidence for the existence of superionic ice we collected last year."
Analyzing how the X-ray diffraction patterns varied for the different experiments probing increased pressure and temperature conditions, the team identified a phase transition to a previously unknown face-centered-cubic (f.c.c.) atomic structure for dense water ice.
"Water is known to have many different crystalline structures known as ice Ih, II, III, up to XVII," Coppari said. "So, we propose to call the new f.c.c. solid form ‘ice XVIII.’ Computer simulations have proposed a number of different possible crystalline structures for superionic ice. Our study provides a critical test to numerical methods."
The team’s data has profound implications for the interior structure of ice giant planets. Since superionic ice is ultimately a solid, the idea of these planets having a uniform rapidly convecting fluid layer no longer holds.
"Because water ice at Uranus and Neptune’s interior conditions has a crystalline lattice, we argue that superionic ice should not flow like a liquid such as the fluid iron outer core of the Earth. Rather, it's probably better to picture that superionic ice would flow similarly to the Earth’s mantle, which is made of solid rock, yet flows and supports large-scale convective motions on the very long geological timescales," Millot said. "This can dramatically affect our understanding of the internal structure and the evolution of the icy giant planets, as well as all their numerous extrasolar cousins."
Co-authors include Antonio Correa Barrios, Sebastien Hamel, Damian Swift and Jon Eggert from LLNL, and Ryan Rygg, previously at LLNL and now at the University of Rochester. The work was partially funded by the Laboratory Directed Research and Development program.
Contact
 Breanna Bishop
Breanna Bishop
[email protected]
(925) 423-9802
Related Links
Nature"Experimental evidence for superionic water ice using shock compression"
Tags
Lasers and Optical S&TLasers
National Ignition Facility and Photon Science
Physical and Life Sciences
Featured Articles