Fueling up hydrogen production
 (Download Image)
(Download Image)
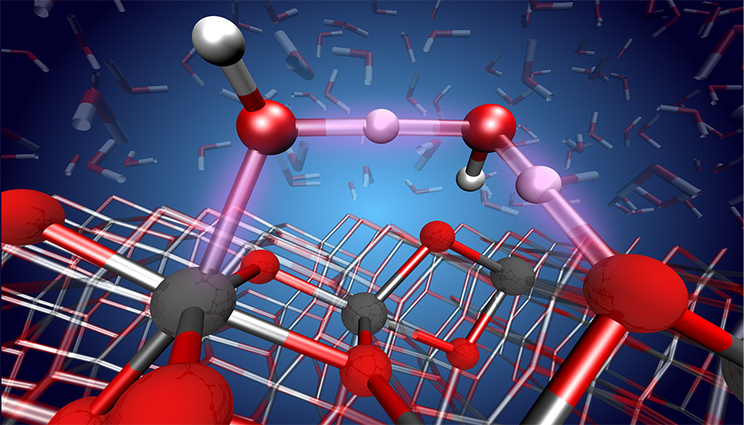
An atomic-level view of the proton transfer reaction between water and the TiO2 surface. Image by Marcos Calegari Andrade/LLNL.
Through machine learning, a Lawrence Livermore National Laboratory (LLNL) scientist has a better grasp of understanding materials used to produce hydrogen fuel.
Water is everywhere in the environment and its interaction with metal oxide surfaces has a key role in processes that range from wetting, dissolution and corrosion to photocatalytic reactions. The relative stability of molecular vs. dissociative adsorbed species can be of critical importance in these processes and has been intensely debated.
The interaction of water with TiO2 (titanium oxide) surfaces is especially important in various scientific fields and applications, from photocatalysis for hydrogen production to photooxidation of organic pollutants to self-cleaning surfaces and bio-medical devices.
TiO2 has for decades being used to accelerate chemical reactions using light. This material was shown to convert water into hydrogen (H2) and oxygen (O2) gases using light, paving the way to the production of clean and renewable energy (since the combustion of hydrogen gas produces water back again).
However, the surface chemistry of TiO2 in contact with water has been the subject of several debates among scientists. The challenge is: if one really wants to understand how water is converted to H2 and O2 by TiO2, one must first understand the chemistry of the region where this reaction takes place.
Marcos Calegari Andrade“It turns out this region is the TiO2 surface,” said Marcos Calegari Andrade, a materials scientist in the Quantum Simulations Group and co-author of a paper appearing in the Proceedings of the National Academy of Sciences. “In our work, we used machine learning to simulate the TiO2 surface in contact with liquid water. Our simulations revealed how sensitive the surface chemistry of TiO2 is relative to the thickness of thin TiO2 films and they provided atomic-level detail into the structure of water close to the TiO2 surface.”
In the new work, LLNL researchers used molecular simulations that accurately reproduce the ab initio results for water interacting with a prototypical TiO2(110) surface. The results have implications for improved production of hydrogen fuel.
“Our fundamental understanding of the TiO2 interface with water will help us to find more efficient and affordable materials to produce hydrogen in a clean and renewable fashion,” Calegari Andrade said.
The work highlights how powerful machine learning is to study chemical reactions at solid-liquid interfaces (such as the TiO2-water interface). Machine learning allows for long and large-scale atomistic simulations with the accuracy of quantum mechanics but at orders of magnitude lower computational costs.
“This will enable us to understand other solid-liquid interfaces that are relevant for renewable energy production,” Calegari Andrade said. “The detailed information of the water interaction with TiO2 is of general interest to people using this material for photocatalysis or even biomedical applications” (since the widely used titanium implants are covered with a thin film of TiO2 on their surfaces).
Researchers from Princeton University, Henan University and Beihang University also contributed to this work. The research was funded by the Department of Energy’s Office of Science, Chemical Sciences, Geosciences & Bioscience Division.
Contact
 Anne M. Stark
Anne M. Stark
[email protected]
(925) 422-9799
Related Links
Proceedings of the National Academy of SciencesDOE Office of Science, Chemical Sciences, Geoscience and Bioscience Division
Tags
Advanced Materials and ManufacturingMaterials Science
Office of Science
Physical and Life Sciences
Featured Articles








