Experiment improves predictions of uranium dispersion
 (Download Image)
(Download Image)
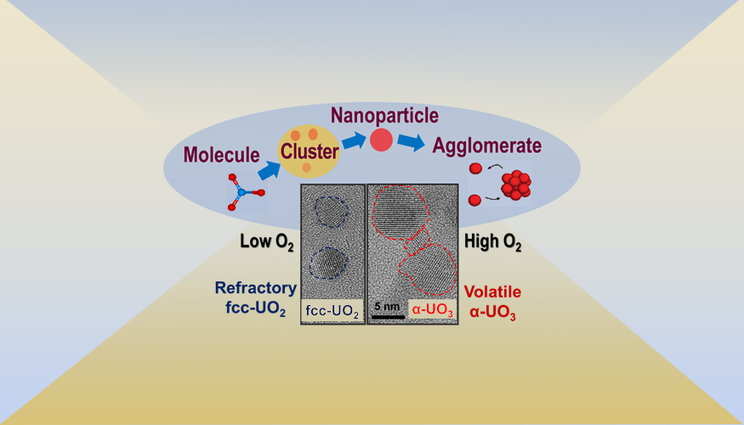
Uranium can prefer to be in metastable oxidation states (i.e., α-UO3) that have higher vapor pressures than refractory forms (i.e., UO2) depending on the oxygen abundance in the surrounding environment and rapidity of the vapor condensation process.
The predictive models that describe the fate and transport of radioactive materials in the atmosphere following a nuclear incident (explosion or reactor accident) assume that uranium-bearing particulates would attain chemical equilibrium during vapor condensation.
In a new study, funded by the Office of Defense Nuclear Nonproliferation Research and Development (DNN R&D) within the U.S. Department of Energy’s National Nuclear Security Administration and the U.S. Department of Defense’s Defense Threat Reduction Agency (DTRA) Basic Science Grant, researchers from Lawrence Livermore National Laboratory (LLNL) and the University of Illinois at Urbana–Champaign (UIUC) demonstrated that kinetically driven processes in a system of rapidly decreasing temperature can result in substantial deviations from chemical equilibrium. This can cause uranium to condense out in metastable oxidation states that have different vapor pressures than the thermodynamically favored oxides, significantly affecting uranium transport.
“This new study will improve our ability to predict uranium’s multiphase transport in nuclear incident scenarios,” said LLNL research scientist Batikan Koroglu, lead author of a paper appearing in Analytical Chemistry.
The physical and chemical processes that occur during the condensation of a nuclear fireball are approximated using fallout models. These models generally assume that atomized elements heated to extremely high temperatures will reach a state of chemical equilibrium as the fireball cools and thermodynamically favored oxides will form once the temperature drops below their boiling points. Uranium oxide is assumed to condense in its most stable form after cooling below its boiling temperature.
However, condensation patterns observed in fallout samples reveal that some fraction of the uranium is “held up” in the vapor phase relative to refractory actinides and fission products.
“This work provides the first, detailed experimental insights able to help explain the long-standing problem of why uranium can exhibit variations in volatile behavior during nuclear fireball condensation -- it’s a big first,” said LLNL nuclear scientist Kim Knight, principal investigator for the DNN R&D effort.
The research team synthesized uranium oxide nanoparticles using a plasma flow reactor under controlled conditions of temperature, pressure and oxygen concentration. They also developed a laser-based diagnostic to detect uranium oxide particles as they formed inside the flow reactor. Using this approach, the researchers gathered direct experimental evidence for a change in the molecular composition of uranium oxide condensates as a function of oxygen concentration. According to the researchers, these results indicate that kinetic models are required to fully describe uranium transport after nuclear incidents.
“Our collaboration with UIUC is part of a DTRA Basic Science Project and allows us to model the data obtained from our plasma flow reactor, which is a unique instrument developed here at the Lab,” says LLNL DTRA principal investigator Harry Radousky. The experimental results are compared to UIUC’s kinetic model describing plasma phase oxidation of uranium. The comparison highlights the competition between the kinetics of gas-phase oxidation of uranium and nucleation of uranium oxide nanoparticles.
Other LLNL scientists supporting the project are Zurong Dai, Michael Armstrong, Jonathan Crowhurst, David Weisz and Timothy Rose.
Contact
 Anne M. Stark
Anne M. Stark
[email protected]
(925) 422-9799
Related Links
Analytical ChemistryOffice of Defense Nuclear Nonproliferation Research and Development
Department of Defense’s Defense Threat Reduction Agency
Tags
Advanced Materials and ManufacturingNuclear, Chem, and Isotopic S&T
Physical and Life Sciences
Materials Science
Nuclear and Chemical Sciences
Featured Articles







